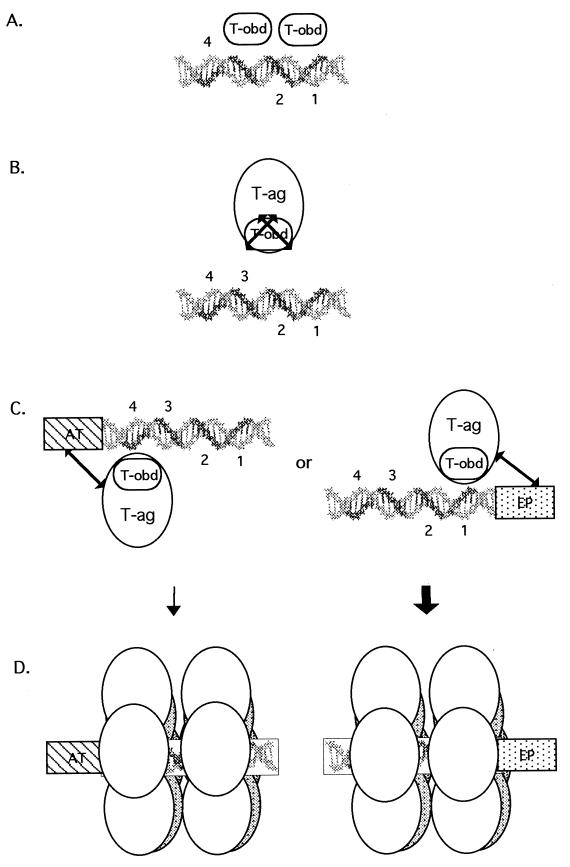
FIG. 11.
Model depicting the requirements for binding of T-ag-obd131–260 and T-ag to site II and the asymmetric extensions of site II. (A) A slightly elongated version of site II is necessary and sufficient for binding of T-ag-obd131–260 (abbreviated T-obd in this model). The GAGGC pentanucleotides are shown in bold and numbered as previously described (43). Based on results obtained in a previous study (39), T-ag-obd131–260 is depicted bound to pentanucleotides 1 and 3 as a dimer (B). In contrast, T-ag and therefore the T-ag-obd present in this molecule cannot bind to oligonucleotides containing just site II. The arrows covering T-ag-obd are used to symbolize that in the context of T-ag, this domain cannot bind to site II. (C) In the presence of either of the flanking sequences, the obstacle(s) to the interactions of T-ag with site II are removed. It is proposed that the flanking sequences enable additional T-ag/origin contacts, depicted by the extended arrows, and that these interactions result in conformational changes that expose T-ag-obd. (D) Upon binding of a T-ag monomer to a pentanucleotide, protein-protein interactions give rise to hexamers; in turn, hexamers promote the cooperative assembly of double hexamers on active pairs of pentanucleotides. The bold arrow is used to show that assembly events involving the EP region are preferred over those involving the AT-rich region (see the text). Finally, while subfragments of the core origin support double-hexamer formation in the presence of AMP-PNP or ADP, only hexamers are detected in the presence of ATP. Therefore, ATP or ATP hydrolysis expands the repertoire of T-ag/core interactions necessary for double-hexamer formation or stability.