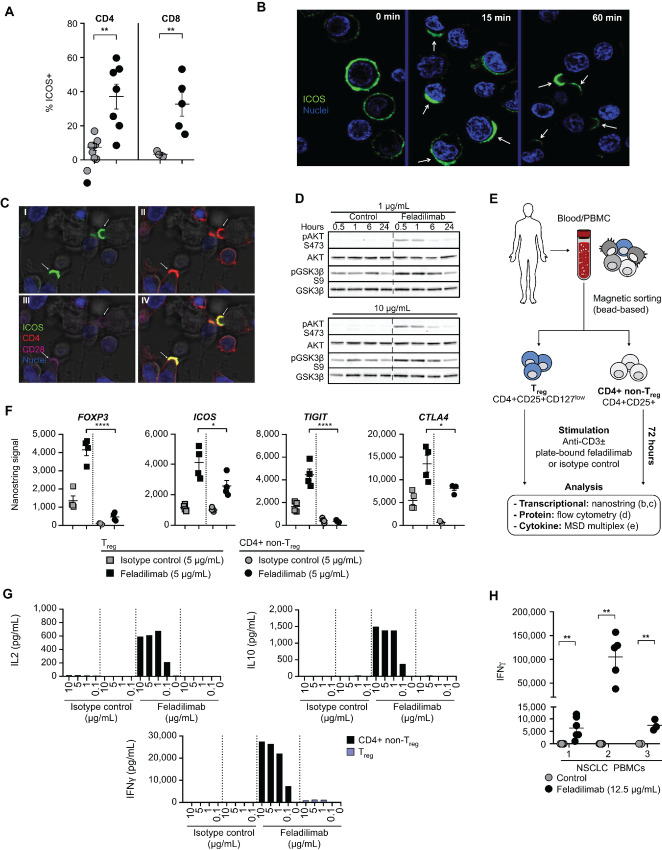
FIGURE 2.
In vitro functional characteristics of antihuman ICOS agonist mAb feladilimab. A, Detection of ICOS on naïve or preactivated (48 hours anti-CD3/CD28) peripheral blood T cells from healthy human donors using feladilimab (3 μg/mL, soluble) and subsequent FITC-conjugated anti-human IgG secondary antibody. Each symbol represents an individual donor. See Supplementary Fig. S1 for flow cytometry gating strategy. Confocal microscopy illustrating kinetics of ICOS cellular localization using feladilimab (3 μg/mL, soluble) as the antibody for staining (B) and T cell–DC interactions following coincubation with feladilimab and CD3/CD28 (C). C, Stimulated T cells exhibit ICOS polarization and mobilization toward neighboring DCs (dark), localizing with related costimulatory receptor CD28; (I–III) denote image overlays, with (IV) combining all markers. D, Representative Western blot analyses of AKT pathway phosphorylation in activated CD4+ T cells following treatment with soluble feladilimab or isotype control (1 and 10 μg/mL, soluble) for 0–24 hours; uncropped images available in Supplementary Fig. S12A. As illustrated in E, CD4+ non-Treg (CD4+ CD25−) and Treg cells (CD4+ CD25+ CD127low) were isolated from healthy donor peripheral blood and stimulated using plate-bound anti-CD3 (1 μg/mL) ± feladilimab or isotype control (each at 5 μg/mL) for 72 hours. F, RNA-based analysis (Nanostring) of Treg-associated marker (FOXP3, ICOS, TIGIT, and CTLA4) expression by the stimulated cell subsets (each symbol represents an individual donor). G, Cytokine-based analysis of T-cell subsets following stimulation with plate-bound anti-CD3 and a dose range of feladilimab or isotype control; see Supplementary Fig. S1 for gating strategy. H, IFNγ production in the supernatant of PBMC cultures from patients with NSCLC following plate-bound feladilimab and anti-CD3 (0.6 μg/mL) stimulation (24 and 48 hours for healthy donors; 72 hours for patients with NSCLC). Data in A and H represent the mean ± s.e.m; significance determined by unpaired Student t test. Where shown, significance was determined by one-way ANOVA. AKT, protein kinase B; Fc, fragment crystallizable; ANOVA, analysis of variance; DC, dendritic cell; ICOS, inducible T cell costimulator; IFN, interferon; Ig, immunoglobulin; mAB, monoclonal antibody; NSCLC, non–small cell lung carcinoma; PBMC, peripheral blood mononuclear cells; s.e.m., standard error of the mean.