Abstract
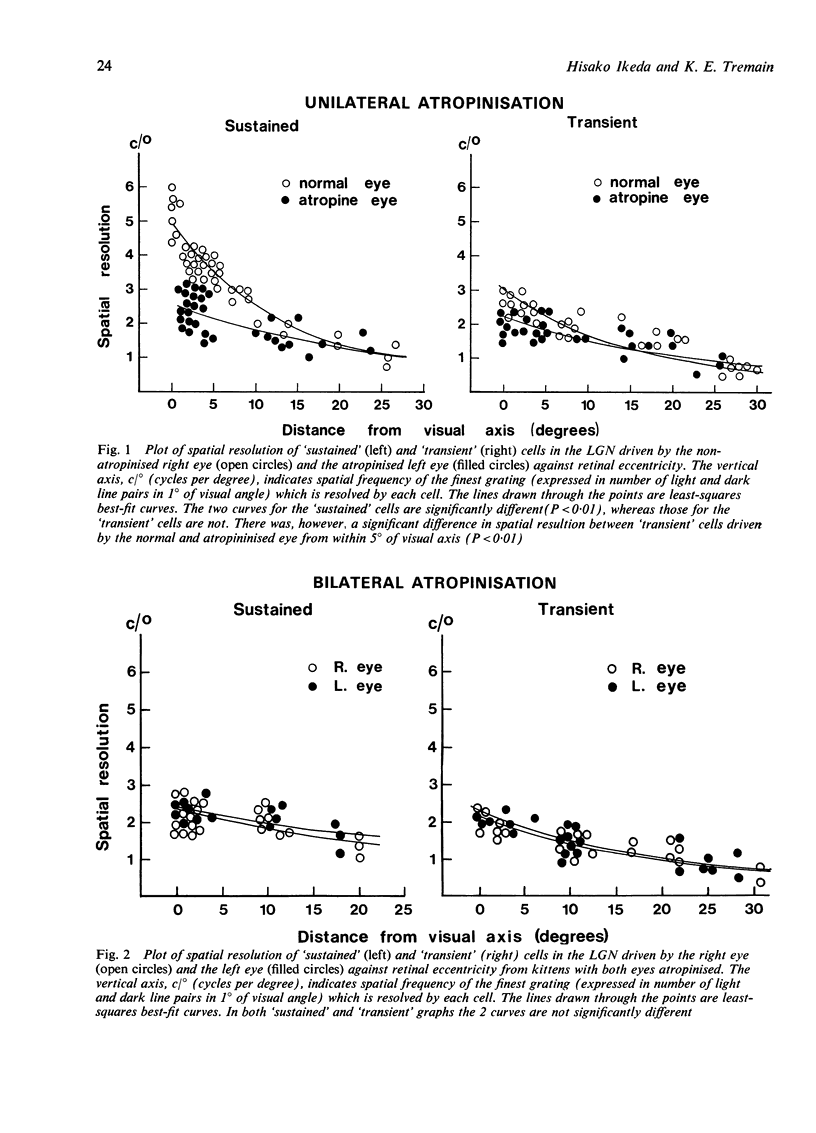
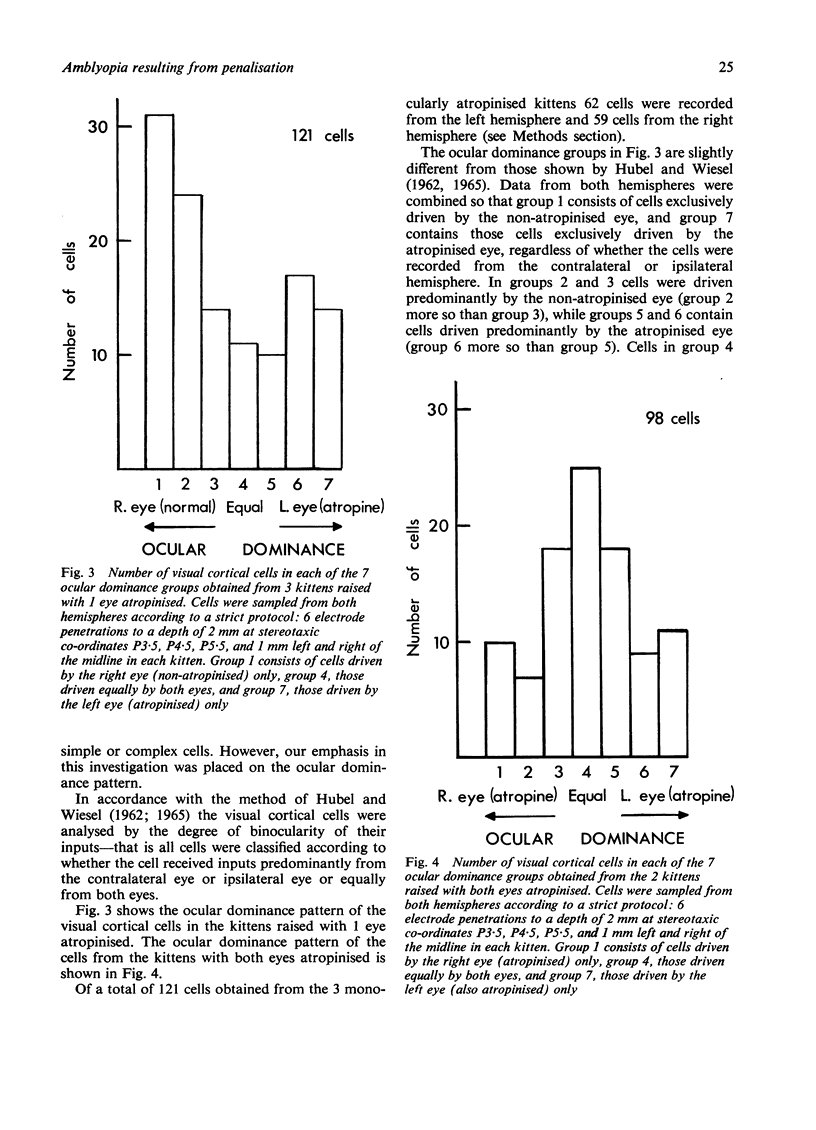
Atropinisation of the eyes--a clinical method of treating strabismus called "penalisation"--in developing kittens caused a reduction in the spatial resolving power of cells in the lateral geniculate nucleus driven by the penalised eye, regardless of whether 1 eye or both eyes had been atropinised. However, binocularity of cells in the visual cortex was reduced only in monocularly penalised cats. It appears that sharply focused foveal images are important in the development of good visual acuity but synergy of the inputs to the 2 eyes is required for the development of binocular vision.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore C. The conditions required for the maintenance of binocularity in the kitten's visual cortex. J Physiol. 1976 Oct;261(2):423–444. doi: 10.1113/jphysiol.1976.sp011566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Experimental analysis of amblyopia and strabismus. Br J Ophthalmol. 1974 Mar;58(3):176–182. doi: 10.1136/bjo.58.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y. Receptive field organization of cat optic nerve fibers with special reference to conduction velocity. Vision Res. 1971 Mar;11(3):209–226. doi: 10.1016/0042-6989(71)90186-6. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Plant G. T., Tremain K. E. Nasal field loss in kittens reared with convergent squint: neurophysiological and morphological studies of the lateral geniculate nucleus. J Physiol. 1977 Sep;270(2):345–366. doi: 10.1113/jphysiol.1977.sp011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tremain K. E. Different causes for amblyopia and loss of binocularity in squinting [proceedings]. J Physiol. 1977 Jul;269(1):26P–27P. [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Differential effects of refractive errors and receptive field organization of central and peripheral ganglion cells. Vision Res. 1972 Sep;12(9):1465–1476. doi: 10.1016/0042-6989(72)90172-1. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Is amblyopia due to inappropriate stimulation of the "sustained" pathway during development? Br J Ophthalmol. 1974 Mar;58(3):165–175. doi: 10.1136/bjo.58.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Properties of LGN cells in kittens reared with convergent squint: a neurophysiological demonstration of amblyopia. Exp Brain Res. 1976 May 10;25(1):63–77. doi: 10.1007/BF00237326. [DOI] [PubMed] [Google Scholar]
- Lombroso C. T., Duffy F. H., Robb R. M. Selective suppression of cerebral evoked potentials to patterned light in amblyopia ex anopsia. Electroencephalogr Clin Neurophysiol. 1969 Sep;27(3):238–247. doi: 10.1016/0013-4694(69)90052-2. [DOI] [PubMed] [Google Scholar]
- Von Noorden G. K. Factors involved in the production of amblyopia. Br J Ophthalmol. 1974 Mar;58(3):158–164. doi: 10.1136/bjo.58.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. EFFECTS OF VISUAL DEPRIVATION ON MORPHOLOGY AND PHYSIOLOGY OF CELLS IN THE CATS LATERAL GENICULATE BODY. J Neurophysiol. 1963 Nov;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Yinon U. Age dependence of the effect of squint on cells in kittens' visual cortex. Exp Brain Res. 1976 Sep 24;26(2):151–157. doi: 10.1007/BF00238279. [DOI] [PubMed] [Google Scholar]
- Yinon U., Auerbach E. The ocular dominance of cortical neurons in cats developed with divergent and convergent squint. Vision Res. 1975 Nov;15(11):1251–1256. doi: 10.1016/0042-6989(75)90170-4. [DOI] [PubMed] [Google Scholar]
- von Noorden G. K. Histological studies of the visual system in monkeys with experimental amblyopia. Invest Ophthalmol. 1973 Oct;12(10):727–738. [PubMed] [Google Scholar]


