FIGURE 2.
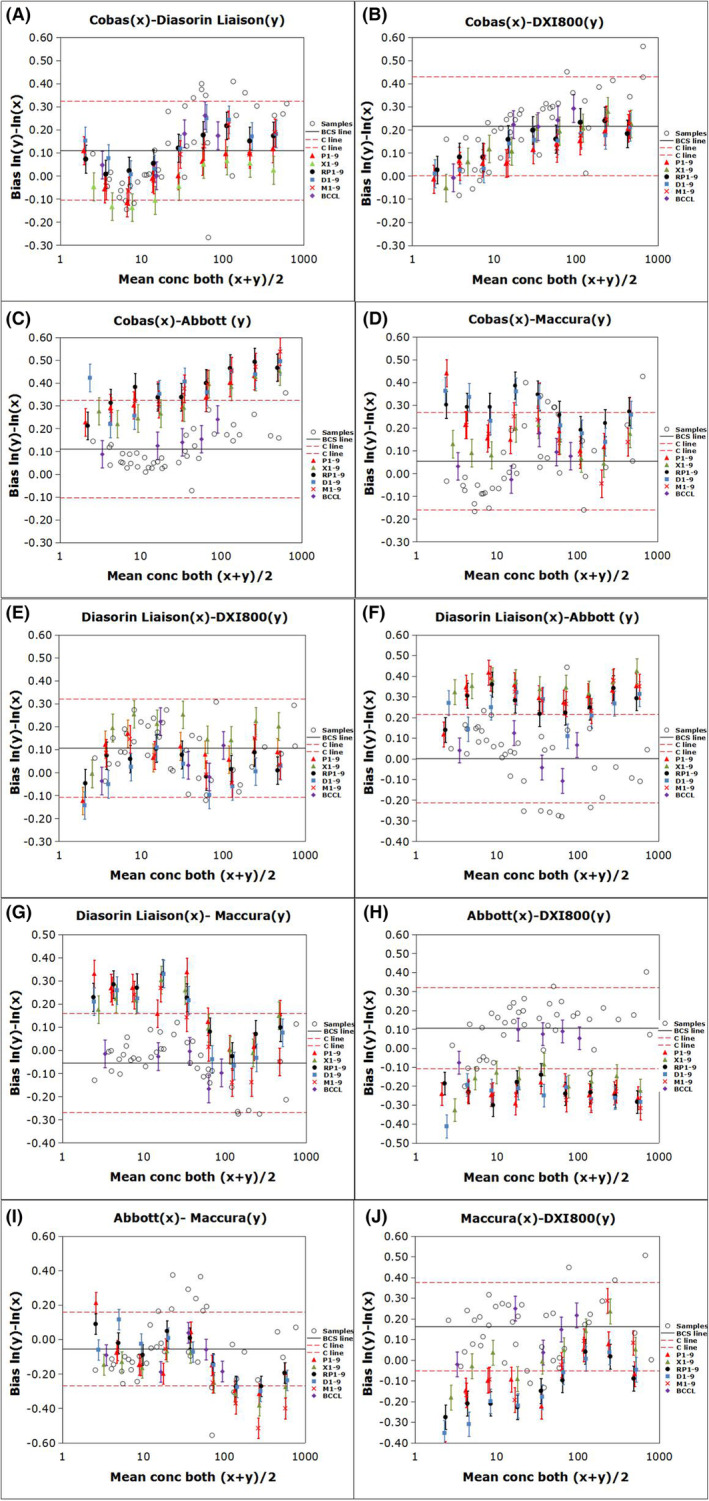
Commutability of the relevant reference materials among the five analytical systems for determining carcinoembryonic antigen (CEA) based on the IFCC method. The bias of the logarithmic transformation of concentration [ln(concentration)] between two measuring systems. The error bars indicate the uncertainty of the difference in bias between each BCCL RM and diluted WHO matrix and the average bias for the clinical samples. The solid black line (BCS line) represents the mean bias for all the clinical samples, and the red dashed lines (C line) indicate the maximum allowable commutability‐related bias.
