Figure 7. Adoptive transfer of bone marrow-derived cells (BMDC) prepared from syngeneic wild-type (WT) mice alleviates proteinuria, kidney injury, and dysfunction in e/e (melanocortin 1 receptor -null) mice with nephrotoxic serum (NTS) nephritis, and reinstates the protective efficacy of melanocortin therapy.
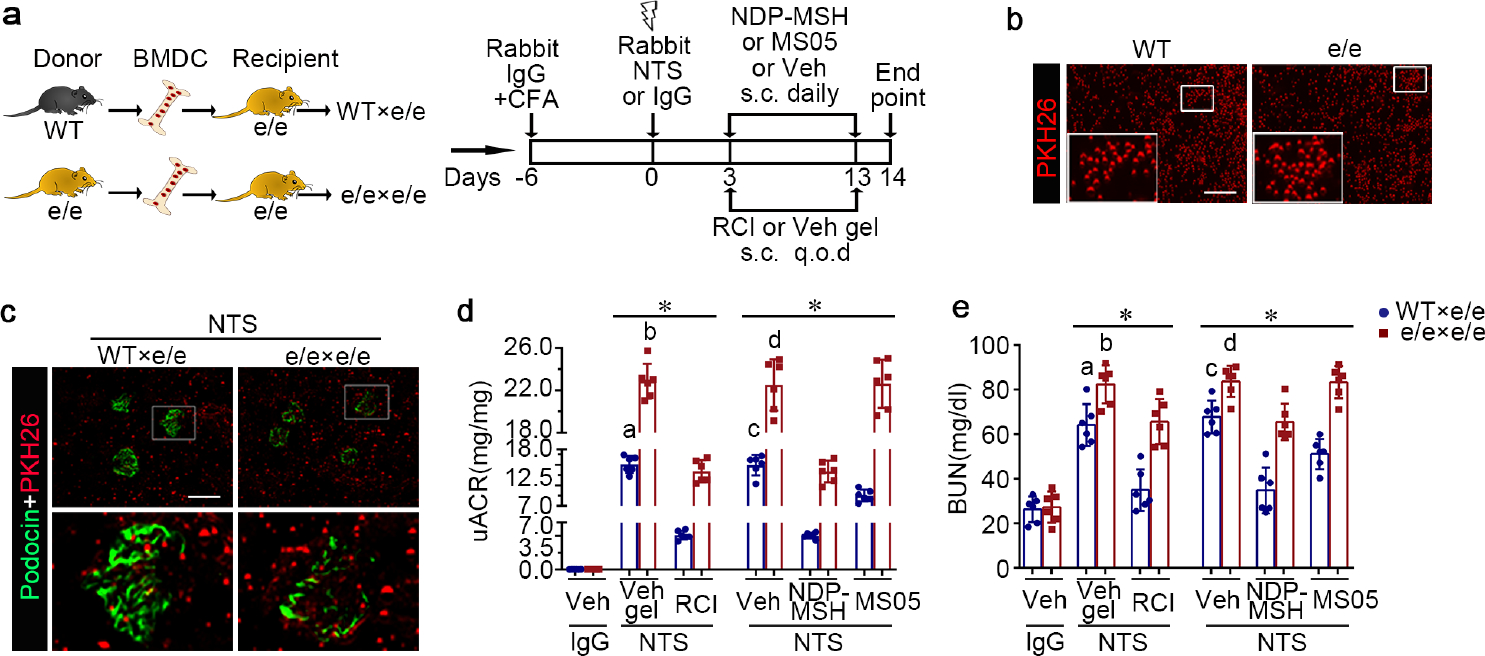
(a) Schematic diagram depicts the animal study design. (b) Representative fluorescent micrographs of red fluorescent dye PKH26-labelled BMDC (red) prior to infusion (bar=100μm). (c) Representative fluorescent micrographs show engraftment of PKH26+ BMDC (red) in renal interstitia and glomeruli, marked by podocin staining (green), in recipient mice 14 days after NTS injury (Scale bar=100μm). (d) Proteinuria was estimated by the urinary albumin-to-creatinine ratios (uACR). (e) Kidney function was assessed by measuring blood urea nitrogen (BUN) levels in sera. *P<0.05 by analysis of variance. aP<0.05 versus NTS-injured mice treated with e/e BMDC and Vehicle (Veh) gel or with WT BMDC and repository corticotropin injection (RCI); bP<0.05 versus NTS-injured mice treated with e/e BMDC and RCI; cP<0.05 versus NTS-injured mice treated with e/e BMDC and Veh or with WT BMDC and pan-melanocortin receptor agonist [Nle, DPhe]-α-MSH (NDP-MSH) or MC1R agonist MS05 (custom-made peptide, GL Biochem); dP<0.05 versus NTS-injured mice treated with e/e BMDC and NDP-MSH; (n=6). CFA, complete Freund’s adjuvant; q.o.d., every other day; s.c., subcutaneous. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
