Figure 8. Adoptive transfer of bone marrow-derived cells (BMDC) prepared from syngeneic wild-type (WT) mice protects e/e (melanocortin 1 receptor -null) mice against nephrotoxic serum (NTS)-elicited podocyte injury, and restores the podocyte protective efficacy of melanocortin therapy.
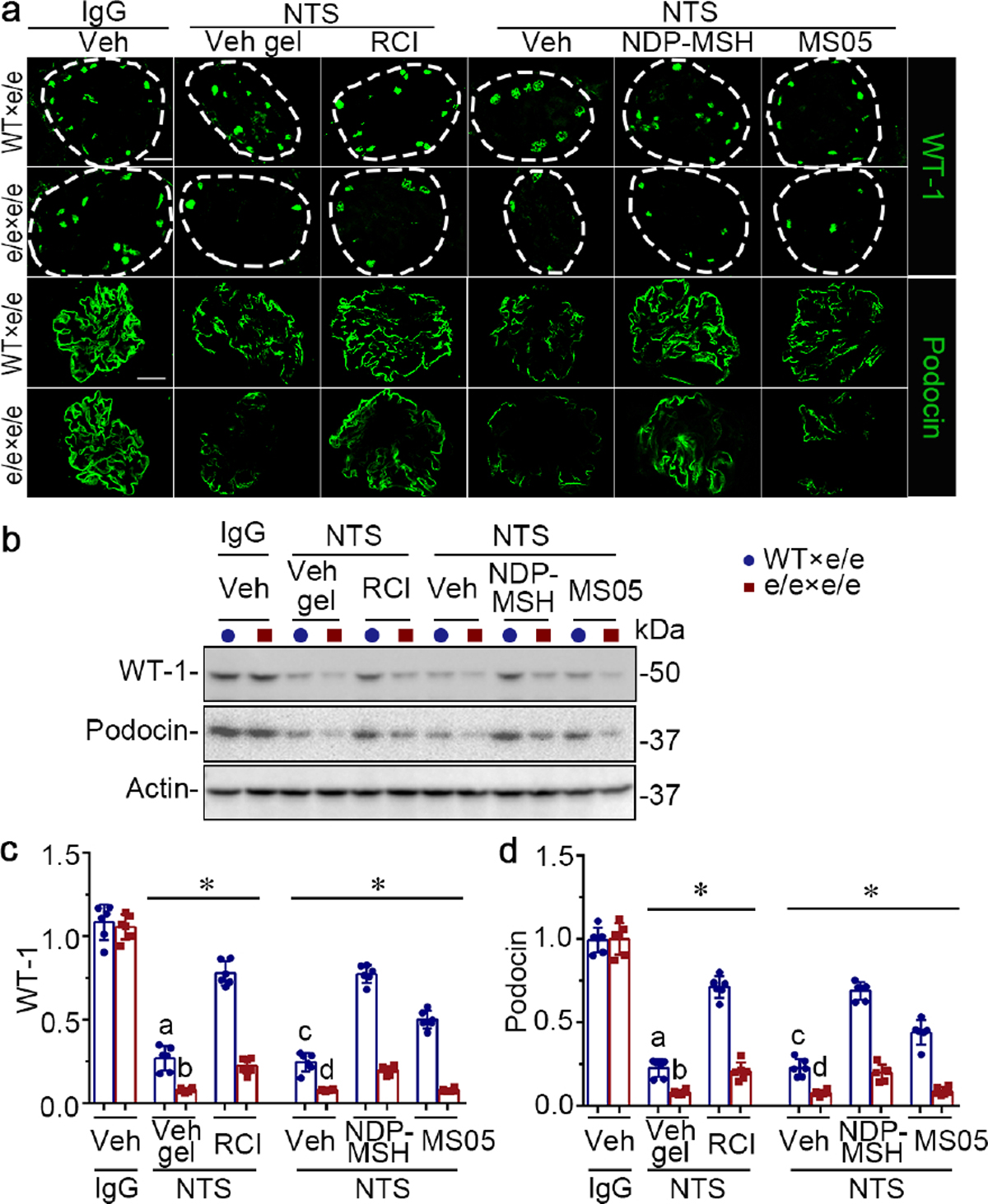
(a) Animals were treated as described in Figure 7. Representative micrographs show immunofluorescence staining of kidney specimens for podocyte-specific markers wild-type (WT) and podocin, with glomeruli outlined by dashed circles (bar =20 μm). (b) Representative blots showing immunoblot analysis of isolated glomeruli for indicated proteins. (c, d) Estimation of the abundance of WT-1 and podocin in glomeruli by densitometric analyses of immunoblots, expressed as relative levels normalized to actin. *P<0.05 by analysis of variance. aP<0.01 versus NTS-injured mice treated with e/e BMDC and Vehicle (Veh) gel or with WT BMDC and repository corticotropin injection (RCI); bP<0.01 versus NTS-injured mice treated with e/e BMDC and RCI; cP<0.05 versus NTS-injured mice treated with e/e BMDC and (Veh) or with WT BMDC and pan-melanocortin receptor agonist [Nle, DPhe]-α-MSH (NDP-MSH) or MC1R agonist MS05 (custom-made peptide, GL Biochem); dP<0.05 versus NTS-injured mice treated with e/e BMDC and NDP-MSH; (n=6). CFA, complete Freund’s adjuvant; q.o.d., every other day; s.c., subcutaneous. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
