Figure 2.
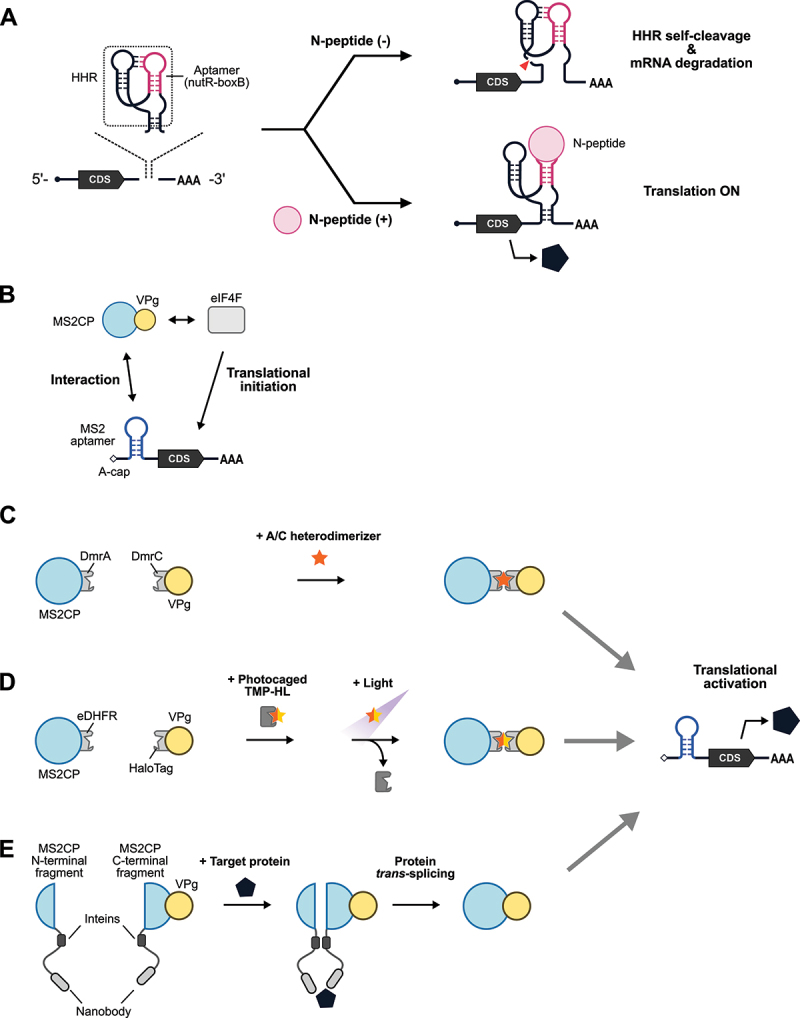
Protein-responsive ON-type translational regulators.
(A) Schematic illustration of protein-responsive riboswitches. A chimera of an aptamer (nutR-boxB hairpin) and a hammerhead ribozyme (HHR) is inserted into the 3’-UTR of mRNA. In the absence of the trigger RNA-binding protein, N-peptide, HHR undergoes self-cleavage, and the mRNA is degraded (translation OFF, top right). In the presence of N-peptide, the binding to nutR-boxB prevents the self-cleavage activity of HHR, resulting in stabilization and translation of the mRNA (translation ON, bottom right). CDS: coding sequence. (B) Schematic illustration of CaVT (Caliciviral VPg-based Translational activator). VPg is capable of interacting with eIF4F to initiate translation. MS2CP fused with VPg interacts with MS2 aptamer inserted into the 5’-UTR of an A-capped target mRNA, recruiting eIF4F proximal to the target mRNA to initiate translation. (C) Schematic illustration of drug-controllable CaVT. A/C heterodimerizer induces the heterodimerization of DmrA and DmrC to tether VPg to the target mRNA for translational initiation. (D) Schematic illustration of light-inducible CaVT. Before light irradiation, photocaged TMP-HL cannot bind to MS2CP-eDHFR. Light irradiation removes the photocage, and a ternary complex of MS2CP-eDHFR, TMP-HL, and HaloTag-VPg activates target mRNA translation. (E) CaVT-based protein sensor. Target proteins act as a molecular hub for MS2CP (N-terminal fragment)-intein nanobody and nanobody-intein-MS2CP (C-terminal fragment)-VPg and enhance intein-mediated protein trans-splicing between these proximal proteins, resulting in reconstitution of full-length MS2CP-VPg.
