Figure 3.
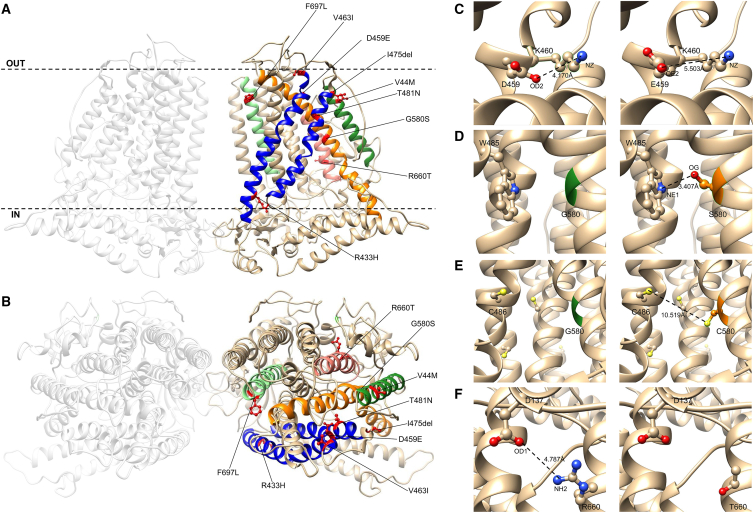
Structural consideration of TMEM63B pathogenic variants
(A and B) View of the predicted tridimensional protein structure of TMEM63B from the membrane plane (A) and the extracellular side (B). All the variants in our cohort map into a transmembrane (TM) helix: p.Val44Met (V44M) in TM1 (dark green helix), p.Arg433His (R433H), p.Asp459Glu (D459E), p.Val463Ile (V463I), p.Ile475del (I475del), and p.Thr481Asn (T481N) in TM4 and TM5 (blue helices), p.Gly580Ser (G580S) in TM7 (orange helix), p.Arg660Thr (R660T) in TM8 (pink helix), and p.Phe697Leu (F697L) in TM9 (light green helix). Dotted line in (A) indicates the plasma membrane, OUT the extracellular side and IN the intracellular (cytoplasmic) side. Details of selected variants are provided in the inlets.
(C) Predicted structural change induced by the D459E substitution. The OD2 atom of Asp459 is predicted to form a buried salt bridge with NZ atom of Lys460 (K460). The substitution of an aspartic acid with a glutamic acid at position 459 increases the distance between the NZ atom of Lys460 and the closer oxygen atom (OE2) available to make a salt bridge, breaking this bond.
(D) Predicted structural change induced by the G580S substitution. The substitution of a glycine (green) with a bulkier amino acid (serine, orange) changes the RSA of the amino acid at position 580 (5.9%–3.8%). In addition, OG atom of Ser580 might form a salt bridge with NE1 atom of Trp485 (W485) and help in stabilizing the structure of the pore.
(E) Predicted structural change induced by the G580C substitution. The substitution of a glycine (green) with a bulkier amino acid (cysteine, orange) changes the RSA of the amino acid at position 580 (5.9%–3.7%). Although the substitution introduces an amino acid with a free SH group that can make disulphide bonds with other amino acids with free SH groups (depicted as yellow spheres), the distance between C580 and the closer amino acid with a free SH group (C486, 10.519 Å) is too big to allow the making of such type of bond.
(F) Predicted structural change induced by the R660T substitution. The substitution of a buried charged residue (arginine) with an uncharged residue (threonine) at position 660 disrupts a salt bridge formed by NH2 atom of Arg660 and Asp137 (D137).