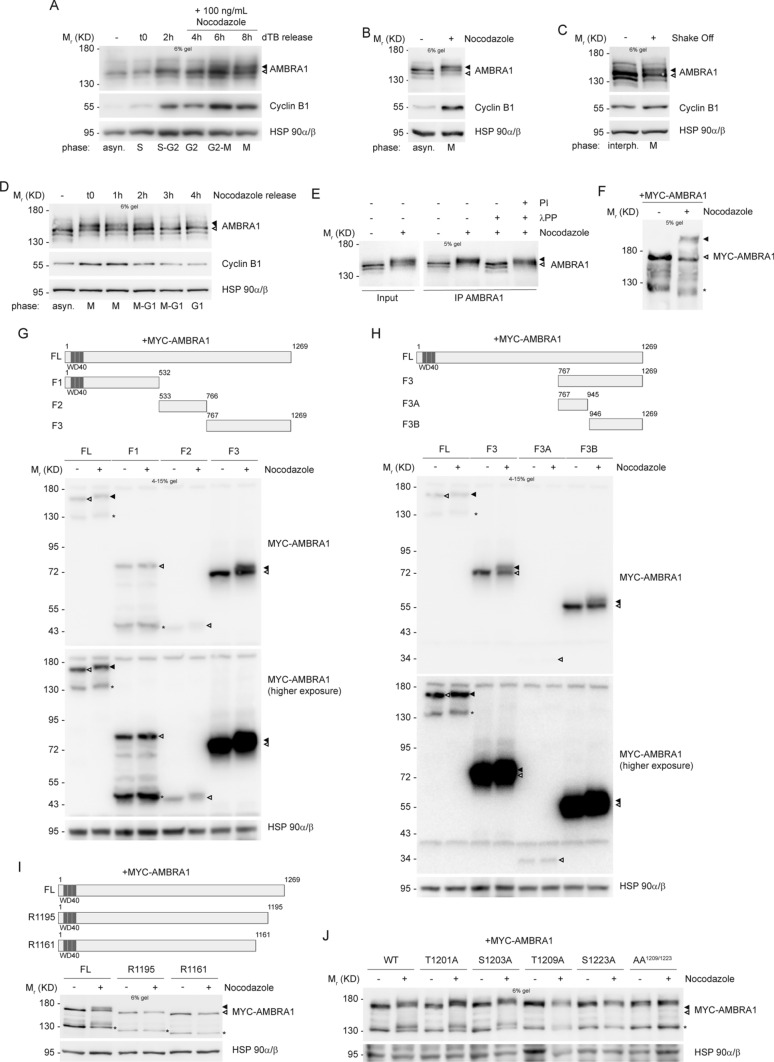
Fig. 1.
AMBRA1 is phosphorylated during mitosis at sites T1209 and S1223. A–D WB analysis of protein extracts from: A HeLa cells synchronized at G1/S boundary with a double Thymidine block (dTB), and released in the presence of 100 ng/mL Nocodazole, to arrest cells in mitosis; B HeLa cells synchronized at mitosis using 200 ng/mL Nocodazole; C HeLa cells in naturally-occurring mitosis after mitotic shake off; D HeLa cells treated with 200 ng/mL Nocodazole, and released. E HeLa cells synchronized at mitosis with Nocodazole, followed by AMBRA1 IP and in vitro phosphatase assay with lambda protein phosphatase (λPP) on immunoprecipitated protein; phosphatase inhibitors (PI) were used as a control. F HeLa cells transfected with MYC-AMBRA1 and then treated with Nocodazole. In this case protein extracts were separated by Phos-Tag SDS-PAGE and then analyzed by WB. G–J WB on protein extracts from: G, H HeLa cells transfected with MYC-AMBRA1 fragments and treated with Nocodazole. MYC-AMBRA1 is showed at a lower (up) and an higher (bottom) exposure. I HeLa cells transfected with MYC-AMBRA1 full-length (FL) or R1161 and R1195 truncated fragments, followed by treatment with Nocodazole. J HeLa cells transfected with MYC-AMBRA1 wild-type (WT) or phosphosilent (Alanine substitution) for T1201, S1203, T1209, S1223 and T1209/S1223 (AA1209/1223). Then cells were treated with Nocodazole. Cell cycle phases are specified for each lane in panels A, B, C and D (asyn. = “asynchronous”; interph. = “interphase”). Schematic representation of truncated fragments respect to full-length protein is provided above WB of panels G, H and I. (FL = “full-length”, F1 = “fragment 1”, F2 = “fragment 2”, F3 = “fragment 3”, F3A = “fragment 3A”, F3B = “fragment 3B”, WT = “wild-type”). WD40 domains (51–90, 93–133, 135–175) are highlighted in dark grey. The white arrow indicates AMBRA1 electrophoretic migration, while the black arrow indicates its mobility shift. AMBRA1 hypershift was visualized with low percentage acrylamide gels (5–6%) or with gradient pre-cast gel for blots in G and H. An asterisk marks a MYC-AMBRA1 degradation sub-product. Gel percentages are indicated in each WB panel