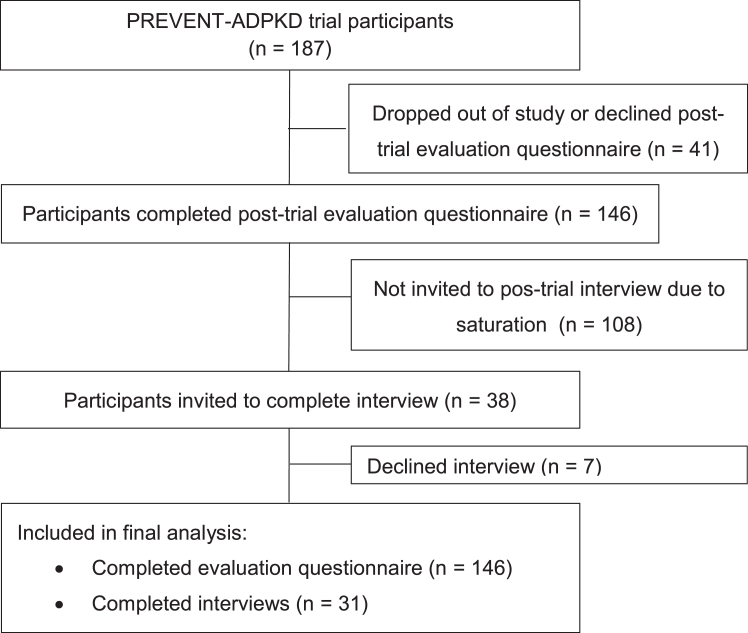
Figure 1.
CONSORT flow diagram showing recruitment of participants with ADPKD for the post-trial questionnaire and semistructured interview. As described in our previous publication8, 158 participants (n=81 in the water intake ad libitum group; n=77 in the prescribed water intake group; reasons for discontinuation of the trial are described in the previous publication). In the current study, an additional 12 participants declined to participate in the post-trial evaluation questionnaire. In other words: 92% (146/158) of participants who completed the initial randomized controlled trial (ie, the PREVENT-ADPKD clinical) agreed to undertake the post-trial questionnaire and 78% (146/187) of participants who were randomized in the initial randomized controlled trial were available for the post-trial questionnaire study.