Abstract
Background
Prevalence of obesity is increasing worldwide. Obesity is associated with incidences of metabolic disorders and cardiovascular diseases and the risk of having it rose sharply during the COVID-19 pandemic. Obesity is associated with oxidative stress, inflammatory markers and hepatic disorders and has become one of the silent killer diseases affecting global health.
Methods
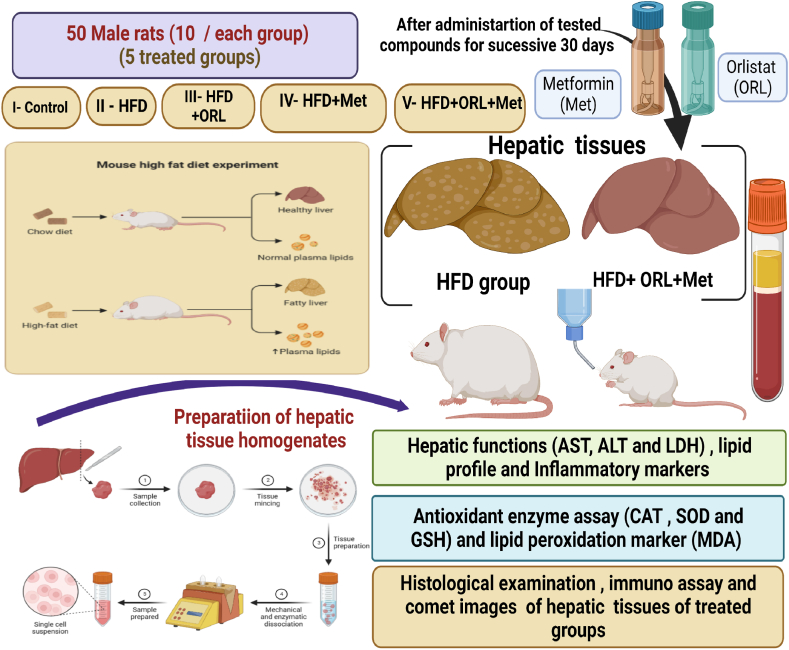
This study examined the effects of obesity on liver functions (ALT, AST and LDH), lipid profile (TG, TC, HDL-c, LDL-c and vLDL-c), tumour necrosis factor alpha (TNF-α), inflammatory marker, C-reactive protein (CRP), leptin hormone and antioxidant enzymes (CAT, SOD and GPx) and lipid peroxidation marker (MDA) in liver homogenates besides histological structure of the liver tissues and assessment of DNA damage. Fifty male Wistar rats were used and they were divided into five treatment groups: I-Control group, II-high-fat diet (HFD) treated group (Obesity) group, III-HFD plus Orlistat (ORL), IV-HFD plus metformin (Met) and V- HFD plus ORL plus Met.
Results
Experimentally-induced obesity caused a significant increase in liver enzymes including lipid markers (triglycerides and total cholesterol), inflammatory markers, tumour markers and lipid peroxidation markers and a concurrent decline in antioxidant enzymes and damage of liver main structures characterised by presence of congestion and accumulation of mononuclear inflammatory cells in blood sinusoids. In contrast, groups treated with either ORL or Met or both group, we recorded restoration of normal hepatic structures and a decline in DNA damage, liver enzymes and antioxidant levels. The best restoration and amelioration were observed in the group treated with a combination of ORL and Met.
Conclusion
Our findings indicated the synergistic effect of ORL and Met in ameliorating hepatic functions and lipid profile, alleviating inflammation, genotoxicity and side effects of experimentally-induced obesity.
Keywords: Obesity, Orlistat, Metformin, Oxidative stress, Diabetes
1. Introduction
Obesity is defined as an excessive amount of body fat in relation to a slope mass that compromises health of individuals [1]. It can lead to incidence of many metabolic disorders such as type 2 diabetes mellitus, dyslipidemias, hypertentions, stroke and coronary artery disease [2,3].
Obesity with incidence of overweight is a pandemic that plays an essential role in the pathogenesis of type 2 diabetes mellitus [4]. The elevation rate of obesity appears to be the first factor for the recent elevation in the incidence of type 2 diabetes mellitus [4,5]. Several medications commonly used for the treatment of obesity and diabetes mellitus with their complications which may elevate the adiposity and may cause metabolic disturbance [[6], [7], [8]]. Obesity is also has a significant role in diabetes mellitus management and an independent risk for cardiovascular complications [4,9]. The prevalence of obesity varies greatly worldwide and in different regions and there is an urgent needs for more data about the effects of obesity and its management in the achievement of metabolic targets in diabetes mellitus patients [10].
Although genetic factors have a major contribution in obesity, over-consumption of a high-fat and high-carbohydrate diet may promote a high energy balance and lead to the development of overweight and obesity status [11,12].
Recently, there has been a constant elevation in the prevalence of obesity in the last three decades. Based on the survey of 2005, prevalence of obesity was estimated to be above 35% [13,14]. Thereafter, following these studies and due to great interest of the Saudi Ministry of Health in alleviation of obesity to avoid it's action that induced several healthy risks, took national measures to control the spread of the obesity epidemic[15].
Metformin (Met) which is an oral drug that is used daily for treatment of type 2 diabetes mellitus. Various mechanisms of action of metformin have been proposed. These include: regulation of AMP-activated protein kinase (AMPK) and the mechanistic target of rapamycin complex 1 (mTORC1) and interaction with mitochondrial ‘Cu’ ions, which may inhibit the mitochondrial function [16].
Met was developed in the 1950s after revealing the lowering ingredient of blood glucose in goat's rue, which is guanidine. Crude Guanidine leads to hepatotoxicity, meanwhile Met is a derivative with confirmed low toxicity [17].
Diabetes mellitus (DM) disease is a significant risk factor for development of different cancers. The vigorous relationship between DM and cancer has been investigated in liver and pancreas. Diabetic patients treated with Met seem to have a lower risk of incidence of cancer than using other therapeutics [18]. Since diabetes mellitus is an independent cancer risk factor, treatment of diabetes mellitus with Met might decline the risk of cancer. However, it is unclear till now if the cancer risk suppressing role of Met is related to a preventive effect of the therapy of cancer risk [19].
The relationship between Met use and declined cancer risk in diabetic patients was suggested in a previous study, which demonstrated a 23% decline in risk of cancer with using of Met [20]. Several previous studies have provided additional evidences for lowering cancer risks in diabetic patients treated with Met. Additionally, several studies have demonstrated that using of Met, have declined the cancer risk in diabetic patients [21].
Orlistat (ORL, tetrahydrolipstatin) is a pancreatic, gastric lipase inhibitor whose primary effect is to decrease the absorption of fat thereby decreasing calories. Its long-term use is also associated with reductions in blood pressure [22].
ORL is one of the few pharmacologic therapeutics available for patients with diabetes mellitus. It helps them in reducing their body weight and improving glycaemic control. There is previous evidence and studies that ORL in conjunction with changing the lifestyle that may achieve greater weight loss than alterations of lifestyle alone [23].
ORL can also reduce the risk of diabetes mellitus. The Canadian Diabetes Association guidelines suggest that if obese and diabetic patients under anti-hyperglycaemic medication also take ORL for about 12 months, body weight is reduced and level of glycated haemoglobin ‘HbA1c’. improves. Additionally, a European guideline encourages obese patients to take ORL in addition to lifestyle changes as an effective way to prevent DM [24].
This study aimed to evaluate the ameliorative synergistic effects of both ORL and Met therapeutics in alleviating hepatotoxicity, oxidative stress and genotoxicity induced by obesity in an experimental rat model and alleviation of obesity metabolic dysfunctions and complications.
2. Materials and methods
2.1. Animals and ethical statement
Fifty male Wistar rats, aged eight weeks and weighing 150–180 g were obtained from the Faculty of Pharmacy (Animal Unit)— Zagazig University. The male rats were housed under hygienic controlled conditions with normal standard diet and water as a negative control group, whereas experimental groups were fed high-fat diets (HFD). This experimental study was performed according to recommendations for animal care and approved by the ethical committee of Zagazig University under ethical approval number ZU-IACUC/1/F/102/2023. Based on ethical approval, the euthanasia was induced by using low dose of Ketamine (9 cc/kg)/Xylazine (7 cc/kg) to prevent any pain for animals without affecting the experimental results.
2.2. Experimental groups
Male rats were randomly divided into five groups of ten male rats each as shown in Fig. 1.
Fig. 1.
Experimental design.
The five treatment groups were as follows:
I- Control group: was given a standard diet with water ad libitum.
II- HFD group: Male rats were fed a high –fat diet containing 26500.00 kcal/kg of calories daily (twice/day) for 30 successive days. The composition of HFD was as follows: 34% Fat, 25% Fibrer, 22% protein, 7% salt, 10% ash, 2% Calcium, HFD was supplemented with vitamin D and some trace minerals such as Cu, I2, Se and Zn.
III- HFD + ORL: Male rats were fed HFD and subsequently administered ORL at a dose of 12 mg/kg [25] for 30 successive days.
IV- HFD + Met: Male rats were fed HFD and then subsequent treatment of Met at a dose of (70 mg/kg) [26] for successive 30 days.
V-HFD + ORL + Met: Male rats were fed HFD and subsequently administered both ORL and Met (Administration of second dose after 1/2 h of the first dose) at the mentioned doses for 30 successive days, as shown in Fig. 1.
2.3. Samples collection
Blood samples were centrifuged at 5000 r.p.m for 15 min to obtain the serum, The serum was used in purified form for further analysis and it was persevered at −20 °C. The experimental male rats were suddenly decapitated after induction of light anesthesia with xylene/ketamine (I.P), and the liver tissues were then removed, 1st part was used for histological and immunostaining, Meanwhile the 2nd part was weighed, and homogenized and used for evaluation of antioxidant enzyme capacities (Fig. 1). The supernatants of tissue homogenates were obtained after centrifugation at 3000g for 15 min at 4 °C, then the freshly collected supernatants were preserved at −20 °C for future analysis.
2.4. Biochemical biomarkers
2.4.1. Measurement of hepatic functions and inflammation markers
After successive 30 days of treatment, some biochemical markers in serum were assessed as the activity of alanine aminotransferase (ALT), aspartate aminotransferase (AST) by using available commercial kits from (Spinreact Co, Spain) according to the instructions. Serum LDH activity was assessed by using LDH kit (GmbH Schigraben, Hannover, Germany).
The ELISA technique was conducted (Ebio-Science) by following the instructions to determine tumor necrosis factor-alpha (TNF-α) and determination of (CRP) C-reactive protein.
2.4.2. Assessment of lipid profile
The serum total cholesterol (TC) and triglycerides (TG) were determined according to Carr et al. [27]. High-density lipoprotein–cholesterol (HDL–c) was determined based on Warnick et al. [28]. Serum low-density lipoprotein–cholesterol (LDL–c) level was determined based on Friedewald [29] using the following formula: LDL-c = ¼ Total cholesterol levels–(Triglyceride concentration/5) – HDL-c concentration, meanwhile VLDL-c = triglyceride/5.
2.4.3. Assessment of oxidative stress markers
A small piece (0.25 g) of the hepatic tissues were homogenized with cold slightly alkaline buffer and centrifuged to get the supernatant that was further used for performing antioxidant assays. Briefly, tissues were perfused with a 50 mM of sodium phosphate buffer (100 mM Na2HPO4/NaH2PO4, pH 7.4), 0.25 M sucrose and 0.1 mM EDTA. Then, hepatic tissues were homogenized in ∼5 mL cold buffer/g of tissues using the homogenizer. The tissue homogenates were centrifuged at 10,000 r.p.m for 1/4 h at 4 °C for estimating enzymatic assays and was centrifuged at 2500 r.p.m for determination of lipid peroxidation level, and the resultant supernatant transferred into clean and sterilized Eppendorf tubes that then preserved into a deep freeze until subsequent used.
Superoxide dismutase enzyme activity (SOD) was assessed as one unit of SOD activity was calculated as the amount of protein caused 50% autooxidation inhibition for pyrogallol according to Marklund and Marklund [30]. Malondialdehyde (MDA) was measured via using 1,1,3,3-tetra ethoxy propane as a standard according to Ohkawa et al. [31].Glutathione peroxidase (GPx) activity was estimated according to the manufacturer instructions [32]. CAT activity was estimated at 240 nm over a period of 3 min according to Aebi [33] (Spectrophotometer SP-2200, Bioespectro). Thiol levels were estimated based on [34]. Protein carbonyl (PC) levels were estimated according to [35], all parameters by using by using Bio-Diagnostic kits.
2.5. Histological and immunohistochemically assessment of liver tissues
Parts of the liver tissues were fixed in neutral buffered formalin (10%) and then embedded in paraffin, then sectioned as well as stained with highly pure hematoxylin and eosin [36]. The sections were examined by using the light microscope and then photographed by using a digital camera. Fixed stained samples of hepatic tissues were examined. Cross sections of the hepatic tissues were examined via the light microscope. The liver slices were blocked with 0.1% mix of (H2O and MEOH) for ∼10–20 min to study proteins related to apoptosis. Hepatic tissues were treated at 4 °C for one night with “polyclonal caspase-3 antibody”. The color intensity of caspase-3 was used as asignificant marker to classify the intensity as the following: (−) means (weak immunostaining), (--++) means (moderate immunostaining), (++++) means (Strong immunostaining), and (---+) means (very weak immunostaining).
2.6. Single cell gel electrophoresis (SCGE) (comet assay)
Small pieces of the liver tissues of control and treated groups were placed into a small Petri dish with an ice-cold mincing solution (Ca2+-and Mg2+-free HBSS, DMSO and EDTA). The viability of the tested cells was determined by analyzing the comet images after electrophoresis according to Endoh et al. [37].
The small hepatic samples were cut into smaller pieces, using a disposable blade. Then, the samples were cut into more finer pieces. The obtained cell suspensions were filtered (100 μm). All hepatic tissues' samples were kept on ice to avoid the light until the end of comet assay procedures. All the comet assay was performed under the alkaline conditions [38]. Briefly, 5 μl of the hepatic cell's suspensions were gently mixed with 120 μl of 0.5% agarose with low melting point at 37 °C and layered onto microscope slides, pre-coated within 1.5% agarose with normal melting point. The microscopic slides were then placed in freshly prepared cold lysing solution overnight and then, placed in electrophoresis cube with alkaline electrophoresis solution (pH > 13) at 4 °C for about 1/4 h. The electrophoresis was performed for 20 min. After electrophoresis, the slides were washed in neutralizing buffer, fixed for few minutes in almost absolute alcohol (96%), then dried, and stored at the room temperature.
2.7. Statistical and data analysis
The data were expressed as mean ± SE., a One-Way Analysis of Variance was employed for comparing several groups, using post-hoc test. Statistical significance at P ≤ 0.05 [39].
We assumed that CAT in hepatic tissue homogenates in HFD group versus HFD + ORL + Met group are 2.29 ± 0.76 versus 4.82 ± 0.75 (U/g). At power 80% and confidence level 95%, sample size is 40. This sample was calculated by OPEN EPI software package [39].
3. Results
3.1. Hepatic functions and lipid profile
Results showed an increase in liver enzymes (AST and ALT) in the HFD male rat group, whereas a combination of ORL and Met afforded potent amelioration in the liver enzymes and restored their levels to almost normal values. These findings confirmed that ORL in combination with Met afforded potent hepatoprotective effects against experimental obesity (Table 1).
Table 1.
Effect of ORL or Met either alone or in combination on liver functions and lipid profile against experimental induced obesity of male rats.
| Parameters | Control | HFD | HFD + ORL | HFD + Met | HFD + ORL + Met |
|---|---|---|---|---|---|
| ALT (U/g) | 12.85 ± 2.02e | 105.69 ± 3.65a | 71.69 ± 4.25b | 57.25 ± 5.69c | 30.25 ± 5.25d |
| AST (U/g) | 14.49 ± 1.39e | 135.25 ± 2.98a | 55.58 ± 3.69b | 35.69 ± 4.25c | 22.39 ± 3.78d |
| LDH (U/g) | 240.68 ± 8.58e | 1456.87 ± 20.65a | 492.58 ± 14.65b | 423.68 ± 15.36c | 320.87 ± 12.69d |
| Total cholesterol (TC) (mg/dL) | 101.58 ± 5.69e | 278.68 ± 7.25a | 156.25 ± 6.52b | 133.25 ± 5.25c | 121.02 ± 5.69d |
| Triglycerides (TG) (mg/dL) | 42.25 ± 2.02d | 94.25 ± 4.25a | 61.25 ± 3.12b | 50.05 ± 2.68c | 45.25 ± 4.08d |
| HDL-c (mg/dL) | 49.52 ± 5.25a | 33.05 ± 3.55e | 44.25 ± 5.25d | 45.98 ± 4.25cd | 47.05 ± 3.26b |
| LDL-c (mg/dL) | 33.58 ± 2.98e | 41.25 ± 3.65a | 36.84 ± 2.65b | 35.78 ± 3.41cd | 34.58 ± 3.65d |
| vLDL-c (mg/dL) | 8.45 ± 1.58e | 18.85 ± 2.02a | 12.84 ± 2.78b | 10.05 ± 1.08cd | 9.05 ± 1.69d |
Means within the same row in each category (mean ± SE) carrying different letters are significant at P ≤ 0.05, where the highest mean value has the symbol (a) and decreasing in value were assigned alphabetically.
ORL: Orlistat; Met:Metformin; HFD: High fat diet; ALT: ALanaine aminotransferase; AST: Aspartame aminotransferase; TC: Total cholesterol; TG: Triglycerides; HDL-c: High density lipoprotein; LDL-c: Low density lipoprotein; vLDL-c: very low density lipoprotein.
There was an increase in cholesterol (TC) and triglycerides (TG) levels in the obesity experimental model with a decrease in HDL-C level and rising of both LDL-c and vLDL-c levels. A combination of ORL and Met mitigated the deleterious effect of both cholesterol and triglycerides and restored the normal values of HDL-c and decreased the levels of LDL-c and vLDL-c.
3.2. Inflammatory marker and tumour necrosis factor
Both TNF-α and CRP were significantly higher in the HFD group than in the normal control group (Table 2). However, other HFD treated groups with either ORL or Met or their combination exhibited a significant decrease in both inflammatory and tumour markers after experimental induction of obesity.
Table 2.
Effect of ORL or Met either alone or in combination on tumor necrosis factor and inflammatory marker against experimental induced obesity of male rats.
| Parameters | Control | HFD | HFD + ORL | HFD + Met | HFD + ORL + Met |
|---|---|---|---|---|---|
| TNF-α (Pg/g) | 11.88 ± 1.22e | 69.58 ± 3.69a | 32.68 ± 2.36b | 27.58 ± 3.69c | 18.69 ± 1.87d |
| CRP (mg/L) | 3.54 ± 2.69e | 36.58 ± 4.25a | 19.87 ± 1.35b | 15.48 ± 1.69c | 11.25 ± 2.98d |
Means within the same row in each category (mean ± SE) carrying different letters are significant at P ≤ 0.05, where the highest mean value has the symbol (a) and decreasing in value were assigned alphabetically.
ORL: Orlistat; Met: Metformin; HFD: High fat diet; TNF-α: Tumour necrosis factor alpha; CRP: C-Reactive protein.
3.3. Oxidative stress biomarkers
Significant biochemical changes were observed in hepatic antioxidant enzymes in experimentally-induced obesity. The results revealed an increase in malondialdehyde in obese animals, indicating an elevation of lipid peroxidation levels (Table 3).
Table 3.
Effect of ORL or Met either alone or their combination on MDA, SOD, CAT and GPx levels against experimental induced obesity of male rats in liver tissues of male rats.
| Parameters | Control | HFD | HFD + ORL | HFD + Met | HFD + ORL + Met |
|---|---|---|---|---|---|
| MDA (μmol/g) | 9.58 ± 1.87 | 49.58 ± 5.69 | 18.58 ± 2.02 | 15.58 ± 3.25 | 11.58 ± 1.69 |
| SOD (U/g) | 5.98 ± 1.64 | 1.20 ± 0.69 | 3.69 ± 1.57 | 4.01 ± 1.23 | 4.56 ± 1.04 |
| CAT (U/g) | 14.02 ± 2.98 | 6.58 ± 1.87 | 8.98 ± 1.25 | 11.58 ± 2.58 | 12.98 ± 2.98 |
| GPx (U/g) | 8.08 ± 1.69 | 2.58 ± 0.98 | 5.25 ± 1.48 | 5.98 ± 1.65 | 6.98 ± 1.87 |
Means within the same row in each category (mean ± SE) carrying different letters are significant at P ≤ 0.05, where the highest mean value has the symbol (a) and decreasing in value were assigned alphabetically.
ORL: Orlistat; Met: Metformin; HFD: High fat diet; MDA: Malondialdehyde; SOD: Superoxide dismutase; CAT: Catalase; GPx: Glutathione peroxidase.
SOD, CAT and GPx activities in liver tissue homogenates were significantly higher in experimentally-induced obesity than in the normal control group. Administration of both ORl and Met improved the antioxidant enzyme activities in obese animals (Table 3). Markedly lower thiol levels (TH) in the liver tissue homogenates in the obese group than in the normal control group were observed. The obesity group treated with a combination of ORL and Met exhibited a significantly higher PC levels than the normal control group (Table 4).
Table 4.
Effect of ORL or Met either alone or their combination on Thiol (SH) and protein carbonyl (PC) against experimental induced obesity of male rats in liver tissues of male rats.
| Parameters | Control | HFD | HFD + ORL | HFD + Met | HFD + ORL + Met |
|---|---|---|---|---|---|
| TH (mmol/g) | 0.83 ± 0.31a | 0.22 ± 0.08c | 0.71 ± 0.23b | 0.75 ± 0.33b | 0.79 ± 0.17b |
| PC (nmol/mg) | 1.41 ± 0.44e | 8.32 ± 1.84a | 2.25 ± 1.28bc | 2.05 ± 1.75c | 1.69 ± 0.95de |
Means within the same row in each category (mean ± SE) carrying different letters are significant at P ≤ 0.05, where the highest mean value has the symbol (a) and decreasing in value were assigned alphabetically.
ORL: Orlistat; Met: Metformin; HFD: High fat diet; TH: Thiol, PC: Protein carbonyl.
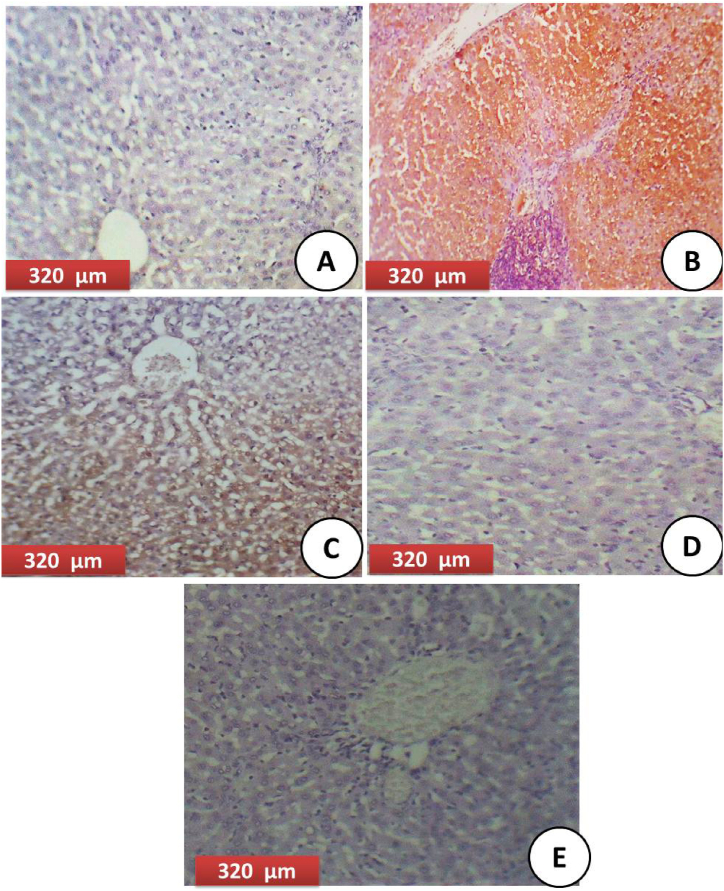
3.4. Histopathology examination of liver tissues
The histological sections of the Control group, showing the appearance of normal hepatic structure with normal sized central vein (Fig. 2A). HFD group of experimental rat liver after experimental induced obesity showing severe toxicity in the form of hypertrophy of hepatocytes with appearance of increased eosinophilia and loss of normal structure with hemorrhage and necrotic tissue (Fig. 2B). HFD plus ORL showing restoration of normal hepatic structure with normal polygonal hepatocytes with moderate congested central vein (Fig. 2C). HFD plus Met showing restoration of normal hepatic structure with moderate congested dilated central vein (Fig. 2D). HFD plus ORL plus Met showing highly restoration of normal hepatic structure with normal hepatocytes with normal staining (Fig. 2E).
Fig. 2.
(A) Control group: showing appearance of the normal hepatic structure with normal sized central vein (CV) (H&EX400). (B) HFD group of experimental rat liver after experimental induced obesity showing severe hypertrophy of hepatocytes with appearance of increased eosinophilia and loss of normal structure (***), granular cytoplasm and vesicular nuclei, the central vein is highly dilated with hemorrhage and appearance of necrotic tissues (Green arrow) with accumulation of few mononuclear inflammatory cells in the blood sinusoids (H&EX400). (C) HFD plus ORL showing restoration of normal hepatic structure with normal polygonal hepatocytes with moderate congested central vein (Green arrow) and loss of normal hepatic structure (H&EX400). (D) HFD plus Met showing restoration of normal hepatic structure with moderate congested dilated central vein (Red and Green arrow) and increment of eosinophilia (H&EX400). (E) HFD plus ORL plus Met showing highly restoration of normal hepatic structure with disappearance of inflammation or congested central vein with normal hepatocytes with normal staining (H&EX400). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The immunohistological sections of the hepatic tissues of the control group showing appearance of (-ve) caspase-3 staining (Fig. 3A), meanwhile, HFD group showing marked immunostaining of caspase-3 (Severe apoptosis) (Fig. 3B). For HFD plus ORL treated group showing very mild staining of caspase-3 (Fig. 3C). Regarding HFD plus Met of the hepatic tissues showing moderate caspase-3 immunostaining (Fig. 3D). Considering HFD plus ORL plus Met of the hepatic tissues showed mild to weak caspase-3 immunostaining (Very weak immunostaining) (Fig. 3E).
Fig. 3.
(A) Control group: Cross-section of the hepatic tissues of the control group showing appearance of (-ve) caspase-3 staining (−) (negative immunostaining) (320 μm). (B): HFD group showing marked immunostaining of caspase-3 (Severe apoptosis) and toxicity in hepatocytes (++++) (very strong mmunostaining) (320 μm). (C) HFD plus ORL showing very mild staining of caspase-3, (--+) (Mild immunostaining) (320 μm). (D) HFD plus Met of the hepatic tissues showing moderate caspase-3 immunostaining in the hepatocytes, (--++) (Moderate immunostaining) (320 μm). (E) HFD plus ORL plus Met of the hepatic tissues showed very mild to weak caspase-3 immunostaining (---+) (Very weak immunostaining) (320 μm) (DAB chromogen, Meyer's hematoxylin counterstain).
The histological scoring was evaluated based on the examination degree of microscopic features in the liver tissues as an effect of ORL and Met alone or in combination (Table 5). A significant inflammation was noticed in HFD induced group as compared with the normal control group. A marked improvement in the hepatocellular scores were observed in treated groups with ORL and/or Met, thus showing its amelioration effect.
Table 5.
Histological activity index (HAI) was assessed based on the degree of microscopic features in liver tissues against experimental obesity of male rats as the effect of ORL and Met either separately or in combination.
| Findings | Groups |
||||
|---|---|---|---|---|---|
| Control | HFD | HFD + ORL | HFD + Met | HFD + ORL + Met | |
| Normal hepatic structure | ++++ | ---+ | --++ | --++ | -+++ |
| Normal sized central vein | ++++ | ------ | -+++ | ---+ | -+++ |
| Congested central vein | ------ | ++++ | --++ | ---+ | ---+ |
| Hypertrophy of hepatocytes | ------ | -+++ | --++ | ---+ | ---+ |
| Hemorrhage and necrotic tissue | ------ | -+++ | ---+ | ---+ | ------ |
| Increased eosinophilia, Toxicity and Inflammation | ------ | ++++ | ---+ | ---+ | ------ |
-----Absence; --- + lesions found in 2–3 rats; --++ lesions found in 5–6 rats; -+++ lesions found in 7 rats, ++++ lesions found in 8 rats at least (n = 8).
Means within the same column in each category (mean ± S.E) carrying different letters are significant at P ≤ 0.05, where the highest mean value has the symbol (a) and decreasing in value were assigned alphabetically.
ORL: Orlistat; Met: Metformin; HFD: High fat diet.
3.5. Comet assay
Comet assay of the hepatic tissues of normal control group showed almost intact nuclei with no appearance of tail (Fig. 4A). The HFD group showed broken DNA strands with a very small head like a comet (Green arrow) and clear shadow area (Blue arrow) (Fig. 4C). HFD + ORL showed intact nuclei with undamaged DNA with moderate appearance of comet shadow (Purple arrow) (Fig. 4B). HFD plus Met revealed improvement of DNA damage with appearance of almost intact cells (Orange arrow) with appearance of shorter tail (Fig. 4D). Meanwhile, treatment with of HFD with combination of ORL and Met improved the percentage of DNA damage and appearance of intact cells with double strands of DNA (Fig. 4E). Table 6 showed the great DNA percentage damage in HFD group as compared with control group with elongating tail moment, while these parameters were declined greatly in obesity group treated with both ORL and Met.
Fig. 4.
Comet images of cells derived from liver of rat of group (A) control group showed clear significant intact nuclei. (B) HFD group showed marked damage with appearance of more than one apoptotic pyknotic cells with large tail. (C) HFD plus ORL showing some intact nuclei with undamaged DNA loop and appearance of other intact nuclei. (D) HFD plus Met showing amelioration of the DNA with very low percentage of damaged DNA and tail. (E) HFD plus ORL and Met showing almost intact cells with very unclear tail shadow and restoring of normal intact DNA strands.
Table 6.
The effect on oxidative DNA damage level (tail length, DNA% and tail moment) and apoptotic cell population (apoptosis %) in liver tissues against experimental induced obesity of male rats treated with ORL and/or Met and their combinations.
| Group | Tail Length (px) | % DNA in Tail | Tail Moment (Unit) | Apoptosis % |
|---|---|---|---|---|
| Control group | 1.01 ± 0.07 | 1.27 ± 0.46 | 0.25 ± 0.06 | 7.33 ± 1.51 |
| HFD | 17.42 ± 2.47 | 45.67 ± 6.54 | 8.63 ± 1.67 | 86.77 ± 6.42 |
| HFD + ORL | 8.85 ± 0.75 | 22.85 ± 2.52 | 0.75 ± 0.17 | 40.11 ± 2.79 |
| HFD + Met | 7.58 ± 1.78 | 17.89 ± 1.65 | 0.51 ± 0.07 | 31.58 ± 3.54 |
| HFD + ORL + Met | 3.06 ± 1.08 | 10.42 ± 1.77 | 0.40 ± 0.14 | 11.41 ± 3.44 |
Means within the same column in each category (mean ± S.E) carrying different letters are significant at P ≤ 0.05, where the highest mean value has the symbol (a) and decreasing in value were assigned alphabetically.
ORL: Orlistat; Met: Metformin; HFD: High fat diet.
4. Discussion
Obesity is a major health problem facing humanity, especially for patients of chronic diseases like diabetes, hypertension and heart complications. This problem was clearly demonstrated during the COVID-19 pandemic due to severe health effects and complications related to obesity and COVID-19 symptoms. Obesity is one of the silent killer diseases and research need to focus on alternative therapies and novel therapeutic combinations effective against the symptoms and complications of obesity and ensuring public health.
Met is an anti-diabetic and anti-obesity oral drug used daily. It was developed in the 1950s after discovering an ingredient in goat's rue that could lower blood glucose with low toxicity. Meanwhile, ORL is a pancreatic and gastric lipase inhibitor whose first effect is to lower the absorption of lipids and calories. So, we aimed to assess the effectiveness of a combination of Met and ORL against obesity complications and in ameliorating of hepatic toxicity. Our findings indicated that the combination of these two drugs was effective and was more potent than each drug alone.
Additionally, our results showed that the administration of high-fat diet induced obesity associated with elevation in body weight, accumulation of adipose tissues and lipid profile disturbances. Obesity also caused the most serious case of fatty liver with a high level of triglycerides. Treatment with ORL significantly lowered the increase in body weight, in agreement with the findings of Jain et al. [40], Broom et al. [41], and Swinburn et al. [42] who confirmed a significant weight reduction in ORL treated group.
The lipid profile for the HFD group exhibited high cholesterol, triglycerides, LDL-c and vLDL-c, but these values were reduced significantly in the HFD group that was treated with either ORL or Met with the highest reduction being recorded in the group treated with a combination of the two drugs. The lipid reduction in latter g group may be due to the effect of ORL as reported previously by Jain et al. [40] who reported a significant reduction in cholesterol concentration and LDL concentration but, in contrast to our findings, they found no change in triglycerides.
Our results further showed a great improvement of HFD group treated with ORL and as in Jain et al. [40], ORL did not induce any changes in serum ALT and AST, which shows that ORL is effective and safe.
Obesity is associated with a high risk of cardiovascular diseases and atherosclerosis [43]. Obesity is associated with an increase in metabolic disturbances such as insulin resistance, hypertension, dyslipidemia and inflammation [44]. Consumption of high fat diets is one of the major risks of developing obesity. In line with the findings of this study [45], demonstrated that the administration of ORL to HFD rats for two months protected against obesity, reduced cardiac antioxidants levels and elevated the MDA levels in rats. These findings corroborate those of this study, specifically showing a good effect of ORL on antioxidant enzymes indexes (SOD, CAT and GPx) and reducing lipid peroxidation level and, thus, alleviation of oxidative stress.
In this study, the rats were fed an HFD to mimic obesity. The HFD rats were treated with ORL generally exhibited elevated levels of cholesterol, triglycerides and an oxidised form of LDL-c [46]. Similarly, total cholesterol levels and triglycerides LDL-c and vLDL-c were markedly higher in the HFD group than in the control group. High levels of lipids, especially LDL-c, can damage cellular contents [47]. ORL reduced the levels these lipids in the obese rats to lower levels than those in the experimental obesity group. These findings confirm that besides reducing body weight, ORL reduces the individual lipid levels owing to its inhibitory effect on lipase activity [48], which decreases absorbance of free fatty acids. The mechanism of action of ORL involves binding to the serine residue of the lipase enzyme, resulting in a decrease in its activity [49].
The decline in HDL levels in the HFD group may be due to atherogenesis induced by experimental obesity, which can also account for high dyslipidaemia and eventual development of atherosclerosis. Although we observed a significant elevation of HDL level in the HFD group treated with ORL, a higher elevation occurred in the HFD treated with both ORL and Met, confirm findings of previous studies [50,51]. These findings suggest a lower atherosclerosis risk in the group treated with a combination of ORL and Met.
Obesity and high-fat diets are as associated with chronic oxidative stress characterised by elevated levels of reactive oxygen species and impairment of oxidant status. Free radicals (reactive species) initiate vascular damage by inducing changes in vascular inflammation markers [52].
Gastric and pancreatic lipases are enzymes that play a key role in the digestion of fats. ORL, which is a semisynthetic lipstatin, ORL is a potent inhibitor of both gastric and pancreatic lipases, with almost no activity against other enzymes as trypsin and amylase. ORL exerts its effect within the gastrointestinal tract. When ORL administered within food containing fats, ORL partially inhibits the hydrolysis of the triglycerides, thus declining the absorption of free fatty acids and these concepts confirm the accuracy of our obtained results [53].
Obesity is greatly linked with chronic oxidative injuries and inflammation, which leads to progression of atherosclerosis. ORL has a favourable effects on weight loss. ORL has the ability to inhibit nuclear factor kappa-B that mediated inflammation and improve the endothelial dysfunction [54]. Thus, ORL could be useful to improve the atherosclerosis in case of obesity. In addition to incidence of atherosclerosis due to disorder of the lipid metabolism [55].
Obesity is known to increase the concentrations of cholesterol precursors, which reflect the biosynthesis of endogenous cholesterol [56]. Additionally, weight reduction declines cholesterol precursors [57,58]. Our current data are consistent with these previous studies, showing that cholesterol concentration tended to decline greatly with treatment with combination of both ORL and Met.
Regarding Met, which is a synthetic biguandie that is mainly absorbed in the small intestine with limited oral bioavailability [59] and this confirm the great improvement in combined treated group with both ORL and Met more than Met alone and this may be due to enhancing the bioavailability of absorption of Met after combination with ORL and increasing its effectiveness.
Confirming the current study concept, Met inhibits the absorption of dietary glucose in rodents and patients with diabetes mellitus [60], this inhibition of glucose is enhanced by administration of Met which resulted from decrease of sodium glucose transporter 1 (SGLIT1) at the membrane of the jejunum with increasing of glucagon –like peptide 1 (GLP1) suggesting some delay in intestinal glucose absorption and this may add explanation to the best results obtained in biochemical reactions with parameters (AST, ALT, Cholesterol, triglycerides, HDL-c and LDL-c) related to treatment with combination of both ORL and Met and elevation of intestinal absorption of both treatment with higher therapeutic efficacy.
An elevation in MDA level is the final and vital indicator of oxidative stress due high lipid peroxidation [61]. In this study, the elevated MDA level accompanied with a decline in SOD, CAT and GPx activities, as well as decreased TH levels in the HFD group, suggests severe oxidative stress, which then accelerated metabolic disturbances and dysfunction. Our findings further showed that treatment of the HFD group with a combination of ORL and Met lowered all oxidative markers and improved hepatic functions, lipid profile, and histological structure.
Several previous studies confirmed that Met can reduce the weight and enhance weight loss in obese animals with or without diabetes mellitus [62,63]. Met may exert its anti-obesity effect via modulating adipose tissue function [64,65]. In obese animals, Met reduces body weight and improves metabolic functions by inhibiting white adipocyte differentiation by retarding fibroblast growth factor 21 (FGF21), which is a vital metabolic hormone that enhances lipolysis and prevents fat accumulation [66]. Consequently, Met is thought to play a great role in preventing weight gain by elevating some molecular markers in a manner that may be dependent or independent of the clinical action of UCP1, a molecular marker that is capable of receding chemical energy into heat [67].
Generally, Met is recognised, not only as a hypoglycaemic agent, but also for its multi effects in combating several metabolic complications including myocardial complications, obesity, insulin resistance, fatty liver and liver dysfunction [68,69]. Several mechanisms by which Met alleviates many metabolic diseases have been proposed. One mechanism involves activation of AMPK, inhibition of the mitochondrial transport chain and alleviation of the glucagon-induced cAMP, eventually leading to the amelioration of glycemic control [70,71].
All the previous studies on Met confirm that it ameliorates metabolic syndromes and alleviates d insulin resistance and corrects lipid profile, consequently, reducing inflammatory markers such as CRP and tumour necrosis factor (TNF-α). The findings of this study corroborate the findings of these previous studies by showing the best biochemical, structural properties and least genotoxicity in the HFD group treated with a combination of both ORL and Met. These results indicate a great potential for synergistic activities of ORL and Met in alleviating obesity complications.
5. Conclusion
Obesity significantly elevated oxidative stress markers, induced hyperlipidemia, inflammation and hepatotoxicity, consequently, causing disruption of hepatic histological architecture, biochemical biomarkers and several metabolic disturbances. A combination of ORL and Met reduced MDA (the final marker of lipid peroxidation), elevated the levels of antioxidant enzymes, improved the lipid profile, reduced inflammatory and tumour markers, improved hepatic histological structures, reduced caspase-3 levels and concurrently restored normal DNA strands and reduced genotoxicity. This study confirmed the potent synergistic effects of ORL and Met in alleviating complications of obesity.
Author contribution statement
Reham Z. Hamza, Khadeejah Alsolami: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
The data that has been used is confidential.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The researchers would like to acknowledge Deanship of Scientific Research, Taif University for funding this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e18724.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lopaschuck G.D., Folmes C.D.L., Stanley W.C. Cardiac energy metabolism in obesity. Circ. Res. 2007;101:335–347. doi: 10.1161/CIRCRESAHA.107.150417. [DOI] [PubMed] [Google Scholar]
- 2.Gordon T., Kannel W.B. Obesity and cardiovascular disease: the framingham study. Clin. Endocrinol. Metabol. 1976;5:367–375. doi: 10.1016/s0300-595x(76)80026-6. [DOI] [PubMed] [Google Scholar]
- 3.Björntorp P. Classification of obese patients and complications related to the distribution of surplus fat. Am. J. Clin. Nutr. 1987;47:1120–1125. doi: 10.1093/ajcn/45.5.1120. [DOI] [PubMed] [Google Scholar]
- 4.Sonmez A., Yumuk V., Haymana C., Demirci I., Barcin C., Kıyıcıd S., Güldiken S., Örük G., Saydam B.O., Baldane S., Kutlutürk F., Küçükler F.K., Deyneli O., Çetinarslan B., Sabuncu T., Bayram F., Satman I. Impact of obesity on the metabolic control of type 2 diabetes: results of the Turkish nationwide survey of glycemic and other metabolic parameters of patients with diabetes mellitus (TEMD obesity study) Obes. Facts. 2019;12:167–178. doi: 10.1159/000496624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bovet P., Chiolero A., Gedeon J. Health effects of overweight and obesity in 195 countries. N. Engl. J. Med. 2017;377(15):1495–1497. doi: 10.1056/NEJMc1710026. [DOI] [PubMed] [Google Scholar]
- 6.Hurren K.M., Dunham M.W. Understanding the impact of commonly utilized, non-insulin, glucose-lowering drugs on body weight in patients with type 2 diabetes. Expet Opin. Pharmacother. 2018;19(10):1087–1095. doi: 10.1080/14656566.2018.1494727. [DOI] [PubMed] [Google Scholar]
- 7.Lau D.C., Teoh H. Impact of current and emerging glucose-lowering drugs on body weight in type 2 diabetes. Can. J. Diabetes. 2015;39(5):S148–S154. doi: 10.1016/j.jcjd.2015.09.090. [DOI] [PubMed] [Google Scholar]
- 8.Domecq J.P., Prutsky G., Leppin A., Sonbol M.B., Altayar O., Undavalli C., et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2015;100(2):363–370. doi: 10.1210/jc.2014-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhan V., Yan R.T., Leiter L.A., Fitchett D.H., Langer A., Lonn E., et al. Guidelines Oriented Approach in Lipid Lowering (GOALL) Registry and Vascular Protection (VP) Registry Investigators. Relation between obesity and the attainment of optimal blood pressure and lipid targets in high vascular risk outpatients. Am. J. Cardiol. 2010;106(9):1270–1276. doi: 10.1016/j.amjcard.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 10.Masmiquel L., Leiter L.A., Vidal J., Bain S., Petrie J., Franek E., et al. Leader 5: prevalence and cardiometabolic impact of obesity in cardiovascular high-risk patients with type 2 diabetes mellitus: baseline global data from the LEADER trial. Cardiovasc. Diabetol. 2016;15(1):29. doi: 10.1186/s12933-016-0341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubert H.B., Feinleib M., McNamara P.M., Castelli W.P. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Study. Circulation. 1983;67:968–983. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 12.Swinburn B., Egger G. Preventive strategies against weight gain and obesity. Obes. Rev. 2002;3:289–301. doi: 10.1046/j.1467-789x.2002.00082.x. [DOI] [PubMed] [Google Scholar]
- 13.Kang J.H., Kang J.Y. Lifestyle measures in the management of gastro-oesophageal reflux disease: clinical and pathophysiological considerations. Ther Adv Chronic Dis. 2015;6(2):51–64. doi: 10.1177/2040622315569501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyson J., Jaques B., Chattopadyhay D., Lochan R., Graham J., Das D., Aslam T., Patanwala I., Gaggar S., Cole M., Sumpter K., Stewart S., Rose J., Hudson M., Manas D., Reeves H.L. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 2014;60(1):110–117. doi: 10.1016/j.jhep.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Memish Z.A., El Bcheraoui C., Tuffaha M., Robinson M., Daoud F., Jaber S., Mikhitarian S., Al Saeedi M., AlMazroa M.A., Mokdad A.H., Al Rabeeah A.A. Obesity and associated factors--Kingdom of Saudi Arabia. Prev. Chronic Dis. 2014;9(11):E174. doi: 10.5888/pcd11.140236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelrahman S., Alghrably M., Campagna M., Hauser C.A.E., Jaremko M., Lachowicz J.I. Metal complex formation and anticancer activity of Cu(I) and Cu(II) complexes with metformin. Molecules. 2021;26:4730. doi: 10.3390/molecules26164730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Congiu T., Alghrably M., Emwas A.H., Jaremko L., Lachowicz J.I., Piludu M., Monica P., Faa G., Pichiri G., Jaremko M., et al. Undercover toxic ménage à trois of Amylin, Copper(II) and Metformin in human embryonic kidney cells. Pharmaceutics. 2021;13:830. doi: 10.3390/pharmaceutics13060830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeCensi A., Puntoni M., Goodwin P., Cazzaniga M., Gennari A., Bonanni B., Gandini S. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev. Res. 2010;3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 19.Chen K., Li Y., Guo Z., Zeng Y., Zhang W., Wang H. Metformin: current clinical applications in nondiabetic patients with cancer. Aging. 2020;12:3993. doi: 10.18632/aging.102787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans J.M., Donnelly L.A., Emslie-Smith A.M., Alessi D.R., Morris A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Y u H., Yin L., Jiang X., Sun X., Wu J., Tian H., Gao X., He X. Effect of metformin on cancer risk and treatment outcome of prostate cancer: a meta-analysis of epidemiological observational studies. PLoS One. 2014;9 doi: 10.1371/journal.pone.0116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aldekhail N.M., Logue J., McLoone P., Morrison D.S. Effect of orlistat on glycaemic control in overweight and obese patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2015;16:1071–1080. doi: 10.1111/obr.12318. [DOI] [PubMed] [Google Scholar]
- 23.Derosa G., Cicero A.F., D'Angelo A., Fogari E., Maffioli P. Effects of 1-year orlistat treatment compared to placebo on insulin resistance parameters in patients with type 2 diabetes. J. Clin. Pharm. Therapeut. 2012;37:187–195. doi: 10.1111/j.1365-2710.2011.01280.x. [DOI] [PubMed] [Google Scholar]
- 24.Avenell A., Broom J., Brown T.J., et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol. Assess. 2004;8:1–182. doi: 10.3310/hta8210. [DOI] [PubMed] [Google Scholar]
- 25.Amin H.M., Tawfek N.S., Abo-El Hussein B.K., Abd El-Ghany M.S. Anti-obesity potential of orlistat and amphetamine in rats fed on high fat diet. Middle East Journal of Applied Sciences. 2015;5(2):453–461. [Google Scholar]
- 26.Zhang S., Xu H., Yu X., Wu Y., Sui D. Metformin ameliorates diabetic nephropathy in a rat model of low-dose streptozotocin-induced diabetes. Exp. Ther. Med. 2017;14:383–390. doi: 10.3892/etm.2017.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carr T., Andressen C.J., Rudel L.L. Enzymatic determination of triglyceride, free cholesterol and total cholesterol in tissue lipid extracts. Clin. Chem. 1993;26:39–42. doi: 10.1016/0009-9120(93)90015-x. [DOI] [PubMed] [Google Scholar]
- 28.Warnick G.R., Benderson J., Albers J.J. Selected methods of clinical chemistry. Amer Assoc Clin Chem. 1983;10:91–99. [Google Scholar]
- 29.Friedewald W.T. Estimation of concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin. Chem. 1982;18:499–502. [PubMed] [Google Scholar]
- 30.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 31.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1970;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 32.Hafeman D.G., Sunde R.A., Hoekstra W.G. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J. Nulr. 1974;104:580–587. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- 33.Aebi H.E. Methods of Enzymatic Analysis. Elsevier; Amsterdam, The Netherlands: 1983. Catalase. [Google Scholar]
- 34.Hu M.L. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994;233:380–385. doi: 10.1016/s0076-6879(94)33044-1. [DOI] [PubMed] [Google Scholar]
- 35.Weber D., Michael J., Davies, Tilman G. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: focus on sample preparation and derivatization conditions. Redox Biol. 2015;5:367–380. doi: 10.1016/j.redox.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabe R.A. Masson Publisher; Paris: 1986. Techniques Histologiques [Histological Techniques] [Google Scholar]
- 37.Endoh D., Okui T., Ozawa S., Yamato O., Kon Y., Arikawa J., Hayashi M. Protective effect of a lignan-containing flaxseed extract against CCl4- induced hepatic injury. J. Vet. Med. Sci. 2002;64(9):761–765. doi: 10.1292/jvms.64.761. [DOI] [PubMed] [Google Scholar]
- 38.Collins A.R., Dunsinka M. In: Methods in Molecular Biology: Oxidative Stress Biomarkers and Antioxidant Protocols. Armstrong D., editor. Humana Press; New Jersy: 2002. Oxidation of cellular DNA measured with the comet assay; pp. pp147–159. [Google Scholar]
- 39.Dean A., Sullivan K., Soe M. 2013. OpenEpi: Open Source Epidemiologic Statistics for Public Health.https://www.OpenEpi.com Available online: [Google Scholar]
- 40.Jain S.S., Ramanand S.J., Ramanand J.B., Akat P.B., Patwardhan M.H., Joshi S.R. Evaluation of efficacy and safety of orlistat in obese patients. Indian Journal of Endocrinology and Metabolism. 2011;15(2):99–104. doi: 10.4103/2230-8210.81938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broom I., Wilding J., Stott P., Myers N., UK Multimorbidity Study Group Randomised trial of the effect of orlistat on body weight and cardiovascular disease risk profile in obese patients: UK Multimorbidity Study. Int. J. Clin. Pract. 2002;56:494–499. [PubMed] [Google Scholar]
- 42.Swinburn B.A., Carey D., Hills A.P., Hooper M., Marks S., Proietto J., et al. Effect of Orlistat on cardiovascular disease risk in obese adults. Diabetes Obes. Metabol. 2005;7:254–262. doi: 10.1111/j.1463-1326.2004.00467.x. [DOI] [PubMed] [Google Scholar]
- 43.Othman Z.A., Zakaria Z., Suleiman J.B., Ghazali W.S.W., Mohamed M. Anti-atherogenic effects of orlistat on obesity-induced vascular oxidative stress rat model. Antioxidants. 2021;10:251. doi: 10.3390/antiox10020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaya A., Koçyiğit C., Çatlı G., Özkan E.B., Dundar B. The relationship between glycemic variability and inflammatory markers in obese children with insulin resistance and metabolic syndrome. J. Clin. Res. Pediatr. Endocrinol. 2017;9:202–207. doi: 10.4274/jcrpe.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Othman Z.A., Noordin L., Omar N., Mohd Yusof N.A., Mohamaed M. Protective effects of orlistat on lipid profile, cardiac oxidative stress biomarkers and histology in high-fat diet-induced obese rats. IIUM Med. J. Malays. 2019;18:1–6. [Google Scholar]
- 46.Monguchi T., Hara T., Hasokawa M., Nakajima H., Mori K., Toh R., Irino Y., Ishida T., Hirata K.I., Shinohara M. Excessive intake of trans fatty acid accelerates atherosclerosis through promoting inflammation and oxidative stress in a mouse model of hyperlipidemia. J. Cardiol. 2017;70:121–127. doi: 10.1016/j.jjcc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Pemberton A.D., Brown J.K. In vitro interactions of Extracellular histones with LDL suggest a potential pro-atherogenic role. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Da Silva R.M., Zarricueta M.L., Moreira D.K.T., De Morais T.R., Rizzardi K.F., Parisotto T.M., Gandra R.L.D.P., Zuin J.C., Caria C.R.E.P., Macedo J.A. Structured lipid containing behenic acid versus orlistat for weight loss: an experimental study in mice. Pharma Nutrition. 2020;14 [Google Scholar]
- 49.Guerciolini R. Mode of action of orlistat. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study. Obes. 1997;21:S12–S23. [PubMed] [Google Scholar]
- 50.Suleiman J.B., Nna V.U., Zakaria Z., Othman Z.A., Abu Bakar A.B., Mohamed M. Obesity-induced testicular oxidative stress, inflammation and apoptosis: protective and therapeutic effects of orlistat. Reprod. Toxicol. 2020;95:113–122. doi: 10.1016/j.reprotox.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Al-Tahami B.A.M., Ismail A.A.A.S., Bee Y.T.G., Awang S.A., Rani W.R.S.W.A., Sanip Z., Rasool A.H.G. The effects of anti-obesity intervention with orlistat and sibutramine on microvascular endothelial function. Clin. Hemorheol. Microcirc. 2015;59:323–334. doi: 10.3233/CH-131765. [DOI] [PubMed] [Google Scholar]
- 52.Steven S., Dib M., Hausding M., Kashani F., Oelze M., Kröller-Schön S., Hanf A., Daub S., Roohani S., Gramlich Y. CD40L controls obesity-associated vascular inflammation, oxidative stress, and endothelial dysfunction in high fat diet-treated and db/db mice. Cardiovasc. Res. 2017;114:312–323. doi: 10.1093/cvr/cvx197. [DOI] [PubMed] [Google Scholar]
- 53.Guerciolini R. Mode of action of orlistat. Int. J. Obes. Relat. Metab. Disord. 1997;21(3):S12–S23. [PubMed] [Google Scholar]
- 54.Kwon Y.J., Kwon G.E., Lee H.S., Choi M.H., Lee J.W. The effect of orlistat on sterol metabolism in obese patients. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.824269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang M., Liu Y., Zhao T., Xiao F., Yang X., Lu B. Dietary sterols and sterol oxidation products on atherosclerosis: an insight provided by liver proteomic and lipidomic. Mol. Nutr. Food Res. 2021 doi: 10.1002/mnfr.202100516. [DOI] [PubMed] [Google Scholar]
- 56.Meuwese M.C., de Groot E., Duivenvoorden R., Trip M.D., Ose L., Maritz F.J. ACAT inhibition and progression of carotid atherosclerosis in patients with familial hypercholesterolemia: the captivate randomized trial. JAMA. 2009;301(11):1131–1139. doi: 10.1001/jama.301.11.1131. [DOI] [PubMed] [Google Scholar]
- 57.Simonen P.P., Gylling H., Miettinen T.A. Body weight modulates cholesterol metabolism in non-insulin dependent type 2 diabetics. Obes. Res. 2002;10(5):328–335. doi: 10.1038/oby.2002.46. [DOI] [PubMed] [Google Scholar]
- 58.Paramsothy P., Knopp R.H., Kahn S.E., Retzlaff B.M., Fish B., Ma L., et al. Plasma sterol evidence for decreased absorption and increased synthesis of cholesterol in insulin resistance and obesity. Am. J. Clin. Nutr. 2011;94(5):1182–1188. doi: 10.3945/ajcn.110.006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foretz M., Guigas B., Viollet B. Mwtformin : update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023:1–17. doi: 10.1038/s41574-023-00833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu T., Xie C., Wu H., Jones K.L., Horowitz M., Rayner C.K. Metformin reduces the rate of small intestinal glucose absorption in type 2 diabetes. Diabetes Obes. Metabol. 2017;19:290–293. doi: 10.1111/dom.12812. [DOI] [PubMed] [Google Scholar]
- 61.Janero D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 62.Ziqubu K., Mazibuko-Mbeje S.E., Mthembu S.X.H., Mabhida S.E., Jack B.U., Nyambuya T.M., Nkambule B.B., Basson A.K., Tiano L., Dludla P.V. Anti-obesity effects of metformin: a scoping review evaluating the feasibility of Brown adipose tissue as a therapeutic target. Int. J. Mol. Sci. 2023;24(3):2227. doi: 10.3390/ijms24032227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee A., Morley J.E. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes. Res. 1998;6:47–53. doi: 10.1002/j.1550-8528.1998.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 64.Dludla P.V., Nkambule B.B., Mazibuko-Mbeje S.E., Nyambuya T.M., Mxinwa V., Mokgalaboni K., Ziqubu K., Cirilli I., Marcheggiani F., Louw J., et al. Adipokines as a therapeutic target by metformin to improve metabolic function: a systematic review of randomized controlled trials. Pharmacol. Res. 2021;163 doi: 10.1016/j.phrs.2020.105219. [DOI] [PubMed] [Google Scholar]
- 65.Yuan T., Li J., Zhao W.G., Sun W., Liu S.N., Liu Q., Fu Y., Shen Z.F. Effects of metformin on metabolism of white and brown adipose tissue in obese C57BL/6J mice. Diabetol. Metab. Syndrome. 2019;11:96. doi: 10.1186/s13098-019-0490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim E.K., Lee S.H., Jhun J.Y., Byun J.K., Jeong J.H., Lee S.Y., Kim J.K., Choi J.Y., Cho M.L. Metformin prevents fatty liver and improves balance of white/Brown adipose in an obesity mouse model by inducing FGF21. Mediat. Inflamm. 2016 doi: 10.1155/2016/5813030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karise I., Bargut T.C., Del Sol M., Aguila M.B., Mandarim-de-Lacerda C.A. Metformin enhances mitochondrial biogenesis and thermogenesis in brown adipocytes of mice. Biomed. Pharmacother. 2019;111:1156–1165. doi: 10.1016/j.biopha.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 68.Yang X., Xu Z., Zhang C., Cai Z., Zhang J. Metformin, beyond an insulin sensitizer, targeting heart and pancreatic β cells. Biochim. Biophys. Acta, Mol. Basis Dis. 2017;1863:1984–1990. doi: 10.1016/j.bbadis.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 69.Adeshirlarijaney A., Zou J., Tran H.Q., Chassaing B., Gewirtz A.T. Amelioration of metabolic syndrome by metformin associates with reduced indices of low-grade inflammation independently of the gut microbiota. Am. J. Physiol. Endocrinol. Metab. 2019;317:E1121–E1130. doi: 10.1152/ajpendo.00245.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nyambuya T.M., Dludla P.V., Mxinwa V., Mokgalaboni K., Ngcobo S.R., Tiano L., Nkambule B.B. The impact of metformin and aspirin on T-cell mediated inflammation: a systematic review of in vitro and in vivo findings. Life Sci. 2020;255 doi: 10.1016/j.lfs.2020.117854. [DOI] [PubMed] [Google Scholar]
- 71.LaMoia T.E., Shulman G.I. Cellular and molecular mechanisms of metformin action. Endocr. Rev. 2021;42:77–96. doi: 10.1210/endrev/bnaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.