Abstract
Iodine 131 (I-131), the principal component of nuclear fallout from the Chernobyl accident, concentrates in the thyroid gland and may pose risks to fetal development. To evaluate this, neonatal outcomes following the accident in April of 1986 were investigated in a cohort of 2582 in utero-exposed individuals from northern Ukraine for whom estimates of fetal thyroid I-131 dose were available. We carried out a retrospective review of cohort members’ prenatal, delivery and newborn records. The relationships of dose with neonatal anthropometrics and gestational length were modeled via linear regression with adjustment for potentially confounding variables. We found similar, statistically significant dose-dependent reductions in both head circumference (−1.0 cm/Gy, P = 0.005) and chest circumference (−0.9 cm/Gy, P = 0.023), as well as a similar but non-significant reduction in neonatal length (−0.6 cm/Gy, P = 0.169). Gestational length was significantly increased with increasing fetal dose (0.5 wks/Gy, P = 0.007). There was no significant (P > 0.1) effect of fetal dose on birth weight. The observed associations of radioiodine exposure with decreased head and chest circumference are consistent with those observed in the Japanese in utero-exposed atomic bomb survivors.
Keywords: Chernobyl, Nuclear accident, Iodine-131, Ukraine, In utero exposure, Neonatal outcomes
Introduction
Pregnant women may be exposed to radiation from medical, occupational or accidental sources. Although radiation is a known teratogen, advice regarding dose restrictions to optimally protect the developing embryo/fetus has been largely limited to pregnant workers and patients [1]. Yet evaluating radiation risks from low dose exposures to pregnant members of the general public is widely recognized as important [2], and the events at Chernobyl and Fukushima underscore the need for more data.
Studies of in utero-exposed survivors of the atomic bombings at Hiroshima and Nagasaki have shown associations between exposure to external ionizing radiation during gestation and harmful effects on the developing brain and on physical growth generally, including reduced head circumference [3] and lower IQ [4] as well as lower weight and shorter stature [5]. These studies were conducted, not among newborns, but among children aged 9–19 years whose prenatal exposure was to external, primarily gamma, radiation. Unlike gamma radiation, iodine-131 (I-131), the principal component of Chernobyl accident fallout, concentrates in the thyroid gland. In those exposed prenatally, during sensitive periods, alterations in thyroid function and/or the hypothalamic-pituitary-thyroid (HPT) axis could potentially affect the developing brain and other organs.
The placenta is freely permeable to iodine, and with the onset of thyroid gland function at 10–12 weeks of gestation, the fetal thyroid begins rapid uptake of iodines, including I-131, passed from the maternal circulation. By late gestation, fetal thyroid iodine uptake is as much as 3–10 times higher than uptake by the mother [6, 7]. Autoregulation of iodine transport to compensate for excess or deficiency does not develop in the fetus until close to term. The fetal thyroid dose resulting from I-131 exposure in utero is thus a function of gestational age at exposure. The timing of exposure also determines the developmental processes that are potentially affected.
Only one previous very small study has been conducted in relation to internal radiation exposure in utero or in early life. Longitudinal measurements of children on the Marshall Islands who were exposed to fallout from Pacific Ocean nuclear testing (38 plus 4 in utero) showed sex and age-dependent effects on growth. Girls were unaffected but boys exposed to radioactive fallout before age five—particularly at 12–18 months—had shorter stature and somewhat lower weight; head circumference was not reduced [8]. The thyroid dose to Marshallese children, however, comes largely from isotopes other than I-131, and from external radiation.
The population exposed due to the Chernobyl accident thus provides a rare opportunity to investigate neonatal effects of in utero exposure to Iodine-131. The study reported here is based on an existing cohort of mother–child pairs in northern Ukraine exposed to I-131 in fallout, assembled initially to assess the risk of thyroid cancer and other thyroid diseases [9]. Our primary aim in this study was to evaluate associations between fetal thyroid I-131 dose and a range of neonatal endpoints, including gestational length and various anthropometric measures. We also examined how these associations vary by trimester at exposure.
Methods
Study population
The Ukraine in utero cohort is comprised of 2582 mother–child pairs in which the woman was pregnant during the period when I-131 from the Chernobyl accident was present in the environment (April 26, 1986 to June 30, 1986). Details of the study design have been reported previously [9]. In brief, an exposed group of mothers was recruited from three northern oblasts (Zhytomir, Chernihiv and Kyiv) considered to be contaminated as a result of the accident (i.e., having Cs-137 deposition levels greater than 37 kBq/m2). The mothers either had direct thyroid radioactivity measurements shortly after the accident or lived in the same contaminated settlement as women of child-bearing age with measured thyroid activity such that doses could be estimated. An unexposed group was comprised of women pregnant during the same period of time and residing in relatively uncontaminated settlements (Cs-137 levels less than or equal to 37 kBq/m2). Standardized procedures for tracing and recruitment resulted in participation rates in excess of 80% among eligible mothers from both exposure groups. Of 1818 women in the contaminated areas found to be eligible, 1494 (82.2%) were successfully enrolled; of 1227 eligible comparison subjects, 1088 (88.7%) agreed to participate.
To collect neonatal endpoint data, we carried out a retrospective review of cohort members’ prenatal, delivery and newborn records at local medical facilities where participating mothers sought care. We also conducted linkage with the Registry at the Institute of Pediatrics, Obstetrics and Gynecology (IPOG) in Kyiv, since many evacuee mothers from contaminated areas ultimately delivered there.
The study was reviewed and approved by the institutional review boards at the National Cancer Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, as well as by the institutional review board at the Institute of Endocrinology and Metabolism (IEM) in Ukraine.
Record retrieval
We began data retrieval by checking the availability of medical records for the period from April 26, 1986 to March 31, 1987 in the obstetric facilities at all places of residence of cohort mothers in 1986. Letters were sent to the administrators informing them about the study and soliciting cooperation. We had personnel from each of 21 local obstetrical facilities retrieve the newborn/delivery records of cohort members, with supervision from collaborating epidemiologists at IEM. The records were brought to IEM and abstracted by a trained team of gynecologists using a standardized form developed jointly from several source documents. After data abstraction, the original records were returned to the medical institutions.
Approximately 30% of the medical records of cohort mothers proved to be irretrievable. Most of this was due to the destruction of records after a mandatory 25-year period of retention, with additional records lost because of floods or fires in institutional archives. In the event of destroyed or lost records, the search for information continued in the hospital’s newborn/delivery registration logs. In addition, we carried out a linkage based on 10 data fields between the demographic database of cohort members and the IPOG Registry database to retrieve information on women from contaminated regions whose delivery dates corresponded to our eligibility criteria and who may have delivered in or near Kyiv. Using the above procedures, we ultimately retrieved data on 2022 cohort members: 57.7% of retrieved data (n = 1167) came from medical records (prenatal, delivery and newborn); 32.6% (n = 659) from registration logs at maternity hospitals; 0.6% (n = 13) from data entered in both records and logs; and 9.1% (n = 183) from linkage with the IPOG Registry.
After the retrieved data were abstracted, we compared the completeness of information on key variables by data source. In all three sources (medical records, registration logs and IPOG Registry), data on birth weight was close to complete (≤5% missing). However, the IPOG Registry files had 100% missing data for gestational length (term of delivery in weeks), as well as for head circumference and chest circumference. The registration logs had 32% missing data for gestational length and 100% missing data for head and chest circumference. In contrast, the medical records had only 7% missing data on gestational length and 22% missing data on head and chest circumference. We compared fetal and maternal characteristics of subjects with data drawn from each of the sources and confirmed that there were no material differences in maternal age at delivery of offspring, maternal weight, parity, gender, or trimester at exposure (not shown). Mean birth weights (~3400 g) and mean gestational age at delivery (~39.7 weeks) were also similar across data sources.
Dosimetry
Individual fetal thyroid doses resulting from intakes of I-131 by the mothers were calculated for offspring members of the cohort by first estimating the variation with time of I-131 activity in the thyroid of the mother and then, using a biokinetic ecologic model, estimating dose to the embryo/fetus [10].
For mothers with direct thyroid measurement (28% of the total), the I-131 activity at the time of measurement was derived from the measurement itself. For mothers from contaminated areas without direct measurements, the age-specific average value of those with measurements was used to infer the I-131 activity in the thyroid at a reference date. For mothers from uncontaminated areas, the I-131 activity in the thyroid was inferred from the relationship observed between Cs-137 activity deposited on the ground and the I-131 activity in the thyroid in settlements with available direct thyroid measurements. The estimate for the mother then served as input to estimating dose to the embryo/fetus based on a bidirectional model developed for the International Commission on Radiation Protection [6]. The model accounts for transfer of iodine between maternal and fetal pools and retention of iodide in the placenta, and predicts a continuous increase in dose with increasing gestational age.
Cumulative I-131 thyroid dose for the full cohort has been estimated to be between 0 and 3230 mGy, with a mean of 72 mGy [10]. Although the thyroid doses received after birth (i.e., postnatal doses) were also estimated, the analyses presented here are based strictly on the prenatal component of the dose. The prenatal doses ranged from 0 to 2263 mGy with a mean of 61 mGy (Table 1).
Table 1.
Distribution of fetal and maternal characteristics
| Full dataset (n = 2022) |
Restricted to medical cards, or medical cards and logs (n = 1180) |
|||||
|---|---|---|---|---|---|---|
| Missing measurement (%) | Mean/median (minimum, maximum) | 1st quartile/3rd quartile | Missing measurement (%) | Mean/median (minimum, maximum) | 1st quartile/3rd quartile | |
| Head circumference (cm) | 1104 (54.6) | 35.55/36 (26, 59) | 35/36 | 262 (22.2) | 35.55/36 (26, 59) | 35/36 |
| Chest circumference (cm) | 1109 (54.8) | 34.67/35 (24, 57) | 34/36 | 267 (22.6) | 34.67/35 (24, 57) | 34/36 |
| Neonatal length (cm) | 239 (11.8) | 51.73/52 (34, 64) | 50/53 | 54 (4.6) | 51.68/52 (34, 64) | 50/53 |
| Birthweight (kg) | 59 (2.9) | 3.40/3.10 (0.00, 5.70) | 0.95/3.80 | 33 (2.8) | 3.40/3.10 (0.00, 5.70) | 0.95/3.80 |
| Gestation length (weeks) | 470 (23.2) | 39.75/40 (27, 48) | 40/40 | 77 (6.5) | 39.72/40 (27, 48) | 39/40 |
| Trimester of exposure (1–3) | 75 (3.7) | 2.07/2 (1, 3) | 1/3 | 58 (4.9) | 2.10/2 (1, 3) | 1/3 |
| Baby sex (male = 0, female = 1) | 242 (12.0) | 0.51/1 (0, 1) | 0/1 | 37 (3.1) | 0.51/1 (0, 1) | 0/1 |
| Singleton versus twin (singleton = 1, twin = 2) | 52 (2.6) | 1.02/1 (1, 2) | 1/1 | 30 (2.5) | 1.02/1 (1, 2) | 1/1 |
| Maternal height (cm) | 1361 (67.3) | 162.49/162 (145, 182) | 158/166 | 519 (44.0) | 162.49/162 (145, 182) | 158/166 |
| Maternal weight at 1st clinic visit (kg) | 1756 (86.8) | 69.15/67 (45, 115) | 61/76 | 914 (77.5) | 69.15/67 (45, 115) | 61/76 |
| Fetal dose (Gy) | 54 (2.7) | 0.061/0.009 (0.000, 2.263) | 0.001/0.046 | 41 (3.5) | 0.046/0.007 (0.000, 1.594) | 0.001/0.031 |
Statistical analysis
The analysis focuses on the relationship between the estimated fetal thyroid I-131 dose and measures of neonatal growth as well as length of gestation. The gestational length variable is based on women’s reports upon arrival at the delivery hospital. Data on birth weight, neonatal length, and head and chest circumference are taken from recorded measurements on newborns. In the sample of 2022 cohort members, analyses were restricted to those with non-missing data on the relevant endpoint as well as fetal dose. Fifty-four subjects with missing prenatal doses were excluded.
We performed linear regression [11] of the continuous outcome measures against fetal iodine dose as the independent variable. Analyses were conducted with and without adjustments for potential confounders, including trimester at exposure, parity, gestational length (for all analyses apart from gestational length), offspring gender, singleton versus twin birth, maternal age at delivery, maternal height and weight at (baseline) first clinic visit. Missing data for these explanatory variables was handled by adding indicator variables for missingness to the regression models. Because of the indications of non-normal distribution of residuals from the fitted linear regression models for three of the endpoints examined (chest circumference, neonatal length, weeks of gestation), we used bootstrap methods, with full resampling of data (rather than residuals) to assess the significance of fit and evaluate confidence intervals on regression parameters of all five endpoints. Bootstrap confidence intervals were derived using the so-called bias-corrected accelerated (BCA) method of Efron [12]. Bootstrap tests of significance, and in some cases for heterogeneity of effect, were also conducted, using standard techniques, for example using bootstrap analogues of the F-test [13]. In a few instances, indicated in the tables, the p value of the bootstrap test of significance did not match the BCA-associated confidence intervals, e.g., the confidence interval excluded 0 but the P value was >0.05. In these cases the P value was adjusted so as to correspond to the indicated CI, via Normal-theory distributional evaluations, so that if the estimated mean is μ and the estimated standard deviation (derived from the relevant CI between 0 and the mean) is SD then
where is the cumulative distribution of the standard normal distribution.
Results
Table 1 shows the mean and median values for outcome variables and covariates, both for the full analytic sample (n = 2022) and for the subset of those with data taken from medical records, or from medical records and logs (n = 1180). Of the 2022 cohort members there were 1104 (54.6%), 1109 (54.8%), 239 (11.8%), 59 (2.9%) and 470 (23.2%) for whom the respective endpoints of head circumference, chest circumference, neonatal length, birth weight and gestational length were missing. The proportion of missing data for all five morbidity endpoints was much less among those with data taken from medical records, or records and logs [head circumference (22.2%), chest circumference (22.6%), neonatal length (4.6%), birth weight (2.8%), gestational length (6.5%)] (Table 1). As can be seen, the distributions of all variables in those with non-missing information are similar in the full analytic dataset and in the subgroup with data taken from medical records or medical records and logs (Table 1).
Table 1 shows that, on average, in the full data set birthweight among cohort offspring was 3400 g with a range from zero to 5700 g, indicating that both low birthweight (<2500 g) and macrosomia (birthweight > 4000 g) were present in a proportion of the cohort. The mean length of gestation was 39.75 weeks—approximately the 40 weeks that is considered full term. The range in gestational length is from 27 to 48 weeks, which includes both preterm (<37 completed weeks) and post-term (≥42 weeks) deliveries, both of which—along with low and high birthweight—carry risks for neonatal morbidities. The sex ratio (female:male) is 51:49.
Tables 2, 3, 4, 5 and 6 present results of the dose–response analyses for each of the neonatal endpoints.
Table 2.
Multivariate regression model to assess the relationship of head circumference (cm) with fetal dose
| Parameter estimate (+95% CI) | 2-sided P value | |
|---|---|---|
| Fetal dose (cm/Gy) | −0.954 (−1.493, −0.329) | 0.005 |
| Trimester 1 versus trimester 3 | −0.214 (−0.524, 0.054) | 0.134 |
| Trimester 2 versus trimester 3 | −0.188 (−0.488, 0.060) | 0.176 |
| Parity ≥1 versus parity 0 | 0.036 (−0.217, 0.267) | 0.771 |
| Gestation weeks (cm/week) | 0.307 (0.214, 0.413) | <0.001 |
| Baby sex female versus baby sex male | −0.307 (−0.543, −0.095) | 0.008 |
| Twin versus singleton | −1.190 (−2.709, −0.284) | 0.010a |
| Maternal age (cm/year) | 0.010 (−2.930, 2.793) | 0.936 |
| Maternal height (cm/cm) | 0.005 (−0.033, 3.470) | 1.000 |
| Maternal weight at 1st clinic (cm/kg) | 0.059 (0.031, 0.138) | 0.010 |
| Model with separate dose × trimester | ||
| Dose in trimester 1 (cm/Gy) | −7.072 (−28.770, 53.512) | 0.660 |
| Dose in trimester 2 (cm/Gy) | −1.504 (−2.326, −0.716) | 0.003 |
| Dose in trimester 3 (or missing) (cm/Gy) | −0.275 (−1.121, 0.867) | 0.541 |
| Linear quadratic model in dose | ||
| Linear dose (cm/Gy) | −1.163 (−2.561, 0.304) | 0.113 |
| Quadratic dose (cm/Gy2) | 0.206 (−0.936, 1.335) | 0.709 |
P value derived from BCA-confidence intervals via Normal distribution
All regressions evaluated using 4999 bootstrap resamples. Model adjusted for trimester of exposure, parity, gestation weeks, baby’s sex, singleton/twin birth, maternal age at delivery of offspring, maternal height and maternal weight at first clinic visit. The estimates for the constant term and for indicators of covariate missingness are not given. Analysis of n = 887 persons with non-missing data on head circumference and fetal dose
Table 3.
Multivariate regression model to assess the relationship of chest circumference (cm) with fetal dose
| Parameter estimate (+95% CI) | 2-sided P value | |
|---|---|---|
| Fetal dose (cm/Gy) | −0.884 (−1.573, −0.103) | 0.023 |
| Trimester 1 versus trimester 3 | −0.017 (−0.357, 0.289) | 0.921 |
| Trimester 2 versus trimester 3 | 0.017 (−0.294, 0.301) | 0.912 |
| Parity ≥1 versus parity 0 | 0.188 (−0.091, 0.459) | 0.178 |
| Gestation weeks (cm/week) | 0.258 (0.165, 0.365) | <0.001 |
| Baby sex female versus baby sex male | −0.360 (−0.602, −0.136) | 0.005 |
| Twin versus singleton | −1.457 (−2.943, −0.409) | 0.025 |
| Maternal age (cm/year) | 0.003 (−3.133, 3.661) | 0.999 |
| Maternal height (cm/cm) | 0.009 (−0.085, 3.254) | 1.000 |
| Maternal weight at 1st clinic (cm/kg) | 0.056 (0.024, 0.131) | 0.018 |
| Model with separate dose × trimester | ||
| Dose in trimester 1 (cm/Gy) | −10.627 (−29.577, 39.067) | 0.291 |
| Dose in trimester 2 (cm/Gy) | −1.592 (−2.310, −0.345) | 0.006 |
| Dose in trimester 3 (or missing) (cm/Gy) | −0.004 (−0.883, 1.212) | 0.994 |
| Linear quadratic model in dose | ||
| Linear dose (cm/Gy) | 0.337 (−1.294, 1.944) | 0.679 |
| Quadratic dose (cm/Gy2) | −1.205 (−2.621, 0.234) | 0.095 |
All regressions evaluated using 4999 bootstrap resamples. Model adjusted for trimester of exposure, parity, gestation weeks, baby’s sex, singleton/twin birth, maternal age at delivery of offspring, maternal height and maternal weight at first clinic visit. The estimates for the constant term and for indicators of covariate missingness are not given. Analysis of n = 882 persons with non-missing data on chest circumference and fetal dose
Table 4.
Multivariate regression model to assess the relationship of neonatal length (crown-heel length, cm) with fetal dose
| Parameter estimate (+95% CI) | 2-sided P value | |
|---|---|---|
| Fetal dose (cm/Gy) | −0.577 (−1.392, 0.259) | 0.169 |
| Trimester 1 versus trimester 3 | −0.176 (−0.456, 0.010) | 0.212 |
| Trimester 2 versus trimester 3 | −0.153 (−0.407, 0.114) | 0.248 |
| Parity ≥1 versus parity 0 | 0.415 (0.178, 0.655) | <0.001 |
| Gestation weeks (cm/week) | 0.597 (0.486, 0.751) | <0.001 |
| Baby sex female versus baby sex male | −0.818 (−1.054, −0.597) | <0.001 |
| Twin versus singleton | −1.789 (−2.606, −0.408) | 0.010 |
| Maternal age (cm/year) | 0.021 (−0.015, 8.900) | 1.000 |
| Maternal height (cm/cm) | 0.069 (0.029, 0.503) | <0.001a |
| Maternal weight at 1st clinic (cm/kg) | 0.021 (−0.042, 0.056) | 0.370 |
| Model with separate dose × trimester | ||
| Dose in trimester 1 (cm/Gy) | 4.241 (−28.123, 28.242) | 0.739 |
| Dose in trimester 2 (cm/Gy) | −0.032 (−1.003, 1.481) | 0.956 |
| Dose in trimester 3 (or missing) (cm/Gy) | −1.120 (−2.673, −0.001) | 0.049a |
| Linear quadratic model in dose | ||
| Linear dose (cm/Gy) | −0.484 (−2.181, 1.372) | 0.597 |
| Quadratic dose (cm/Gy2) | −0.100 (−1.684, 1.468) | 0.902 |
P value derived from BCA-confidence intervals via Normal distribution
All regressions evaluated using 4999 bootstrap resamples. Model adjusted for trimester of exposure, parity, gestation weeks, baby’s sex, singleton/twin birth, maternal age at delivery of offspring, maternal height and maternal weight at first clinic visit. The estimates for the constant term and for indicators of covariate missingness are not given. Analysis of n = 1733 persons with non-missing data on neonatal length and fetal dose
Table 5.
Multivariate regression model to assess the relationship of birthweight (g) with fetal dose
| Parameter estimate (+95% CI) | 2-sided P value | |
|---|---|---|
| Fetal dose (g/Gy) | 42.700 (−78.439, 162.354) | 0.468 |
| Trimester 1 versus trimester 3 | 13.394 (−37.677, 64.555) | 0.608 |
| Trimester 2 versus trimester 3 | −24.789 (−74.108, 22.302) | 0.316 |
| Parity ≥1 versus parity 0 | 126.590 (78.227, 171.512) | <0.001 |
| Gestation weeks (g/week) | 148.561 (130.332, 164.653) | <0.001 |
| Baby sex female versus baby sex male | −153.096 (−195.389, −111.783) | <0.001 |
| Twin versus singleton | −746.407 (−895.443, −589.520) | 0.015 |
| Maternal age (g/year) | 7.604 (1.855, 14.107) | 0.038 |
| Maternal height (g/cm) | 13.258 (7.027, 20.738) | <0.001a |
| Maternal weight at 1st clinic (g/kg) | 10.933 (5.710, 16.624) | <0.001 |
| Model with separate dose × trimester | ||
| Dose in trimester 1 (g/Gy) | −1363.489 (−7392.693, 1216.432) | 0.425 |
| Dose in trimester 2 (g/Gy) | 25.227 (−144.218, 261.346) | 0.806 |
| Dose in trimester 3 (or missing) (g/Gy) | 52.012 (−104.508, 196.065) | 0.485 |
| Linear quadratic model in dose | ||
| Linear dose (g/Gy) | 6.577 (−256.819, 252.871) | 0.963 |
| Quadratic dose (g/Gy2) | 31.374 (−146.373, 226.048) | 0.701 |
P value derived from BCA-confidence intervals via Normal distribution
All regressions evaluated using 4999 bootstrap resamples. Model adjusted for trimester of exposure, parity, gestation weeks, baby’s sex, singleton/twin birth, maternal age at delivery of offspring, maternal height and maternal weight at first clinic visit. The estimates for the constant term and for indicators of covariate missingness are not given. Analysis of n = 1910 persons with non-missing data on birthweight and fetal dose
Table 6.
Multivariate regression model to assess the relationship of gestational age at delivery (weeks) with fetal dose
| Parameter estimate (+95% CI) | 2-sided P value | |
|---|---|---|
| Fetal dose (weeks/Gy) | 0.471 (0.198, 0.836) | 0.007 |
| Trimester 1 versus trimester 3 | −0.165 (−0.375, 0.034) | 0.110 |
| Trimester 2 versus trimester 3 | −0.029 (−0.204, 0.149) | 0.747 |
| Parity ≥1 versus parity 0 | 0.190 (0.009, 0.372) | 0.040 |
| Baby sex female versus baby sex male | −0.041 (−0.188, 0.117) | 0.596 |
| Twin versus singleton | −0.561 (−1.429, 0.153) | 0.164 |
| Maternal age (weeks/year) | −0.012 (−0.034, 0.010) | 0.407 |
| Maternal height (weeks/cm) | 0.009 (−0.011, 0.031) | 0.513 |
| Maternal weight at 1st clinic (weeks/kg) | 0.015 (−0.005, 0.029) | 0.087 |
| Model with separate dose × trimester | ||
| Dose in trimester 1 (weeks/Gy) | 11.870 (−1.732, 42.399) | 0.194 |
| Dose in trimester 2 (weeks/Gy) | 0.213 (−0.538, 0.866) | 0.508 |
| Dose in trimester 3 (or missing) (weeks/Gy) | 0.564 (0.263, 1.017) | 0.009 |
| Linear quadratic model in dose | ||
| Linear dose (weeks/Gy) | 1.230 (0.369, 2.135) | 0.011 |
| Quadratic dose (weeks/Gy2) | −0.630 (−1.297, −0.065) | 0.029a |
P value derived from BCA-confidence intervals via Normal distribution
All regressions evaluated using 4999 bootstrap resamples. Model adjusted for trimester of exposure, parity, baby’s sex, singleton/twin birth, maternal age at delivery of offspring, maternal height and maternal weight at first clinic visit. The estimates for the constant term and for indicators of covariate missingness are not given. Analysis of n = 1506 persons with non-missing data on gestational age at delivery and fetal dose
For head circumference (HC), Table 2 and Fig. 1 demonstrate a highly significant dose-related reduction of −0.954 cm/Gy (95% CI −1.493, −0.329, P = 0.005). The decrement in HC is greatest for those exposed in the first trimester, although the interaction (reduction of head circumference per unit dose by trimester) is not statistically significant (P = 0.242). All individuals with head circumference data came from the representative subset whose data were taken from medical records or records and logs. Hence there is an identical decrement in head circumference with dose in this group (Appendix, Table 7).
Fig. 1.
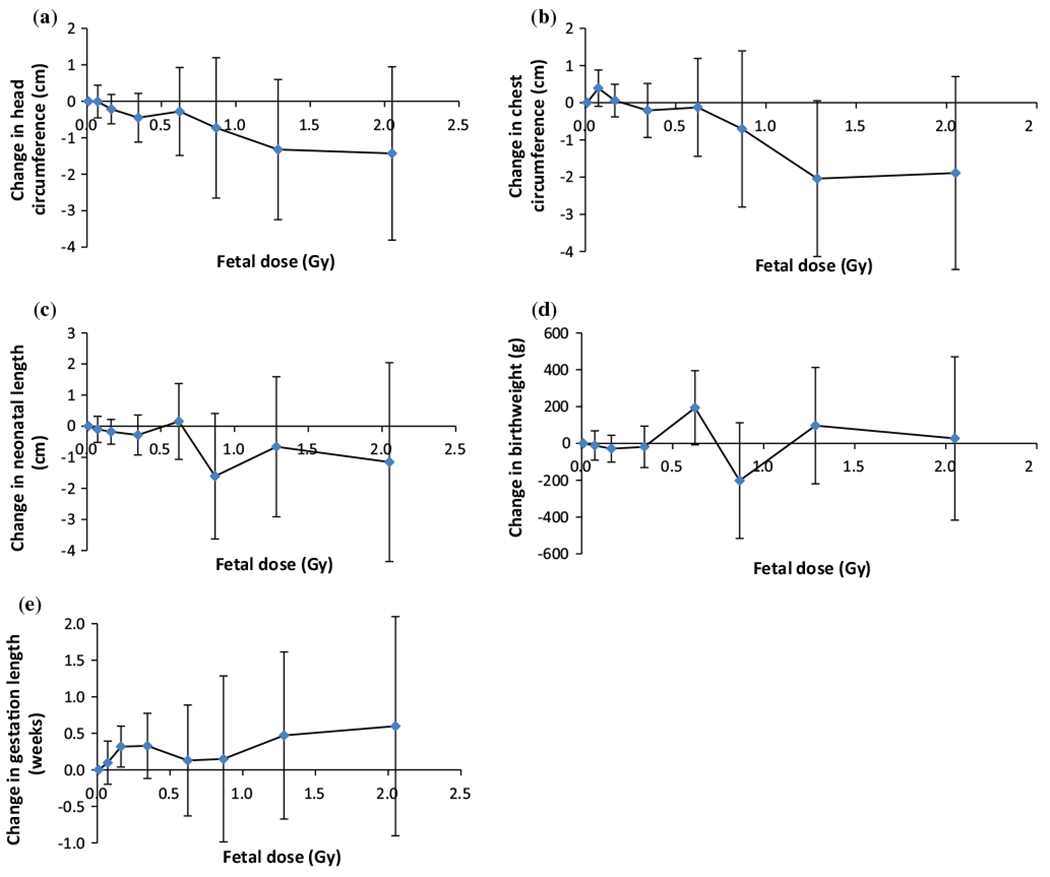
Change from measure in <0.05 Gy fetal dose group in a head circumference, b chest circumference, c neonatal length, d birthweight, and e gestation length. In all cases the baseline is adjusted in the same way as the corresponding variables in Tables 2, 3, 4, 5 and 6
In Table 3 and Fig. 1 we present results from the regression model for the effect of fetal dose on chest circumference, showing a significant dose-related reduction in chest size of −0.884 cm/Gy (95% CI −1.573, −0.103, P = 0.023). As in the case of head circumference, the decrement is greatest for those exposed in the first trimester, but the interaction of reduction in chest circumference per unit dose by trimester does not reach statistical significance (P = 0.144). Again, since all individuals with chest circumference data came from the subset of data taken from medical records or medical records and logs, the results are identical to those in the full dataset (Appendix, Table 8).
Table 4 and Fig. 1 give results from the regression model for neonatal length and fetal dose. In this case, not all subjects with neonatal length data are from the medical record subset. In the full dataset, there is no significant association between dose and neonatal length overall (−0.577 cm/Gy, 95% CI −1.392, 0.259, P = 0.169), although there is a stronger, borderline significant reduction in neonatal length with exposure in the 3rd trimester (−1.120 cm/Gy, 95% CI −2.673, −0.001, P = 0.049). In the medical record subset, there is a similar, non-significant decrement in neonatal length with radiation dose (−0.561 cm/Gy, 95% CI −1.489, 0.524, P = 0.253) (Appendix, Table 9), with indications of a much stronger effect among those exposed in the first trimester, for whom the associations is again borderline statistically significant (P = 0.045).
The regression analyses of Table 5 and Fig. 1 show that in the full dataset there was a small positive, non-significant association between fetal dose and birth weight, with a very wide confidence interval (42.700 g/Gy, 95% CI −78.439, 162.354, P = 0.468). In the subset of data taken from medical records or records cards and logs there is a borderline significant indication of a decrease in birthweight with increasing dose (−135.309 g/Gy, 95% CI −275.303, 25.310, P = 0.075) (Appendix, Table 10), with suggestions of a stronger negative effect among those exposed in the first and third trimesters, the latter borderline statistically significant (P = 0.061).
Table 6 and Fig. 1 demonstrate a highly significant association between fetal dose and later delivery (0.471 weeks/Gy, 95% CI 0.198, 0.836, P = 0.007). Exposures in the 1st trimester had a much stronger effect on the timing of delivery than those later in pregnancy, although only for third trimester exposure was the increase in gestational length conventionally significant (0.564 weeks/Gy, 95% CI 0.263, 1.017, P = 0.009). There is a slightly larger increase in gestational length with radiation dose in the subset of data taken from medical records or medical records and logs (0.787 weeks/Gy, 95% CI 0.273, 1.426, P = 0.014) (Appendix, Table 11), and again there are indications of a much stronger effect among those exposed in the first trimester, although only for third trimester exposure was the increase in gestation length conventionally significant (1.330 weeks/Gy, 95% CI 0.560, 2.710, P = 0.036).
Discussion
In a cohort of individuals exposed prenatally to Chernobyl fallout in Ukraine, the most striking findings were the inverse associations between fetal thyroid I-131 dose and head and chest circumference (estimated reductions of −1.0 cm/Gy). There was also a positive association with gestational length (0.5 wk/Gy). The observed associations with prenatal exposure to I-131 are highly significant and are robust, remaining significant after adjustment for potentially confounding variables. There is no significant effect of fetal thyroid I-131 dose on neonatal length or birthweight.
Although there are important differences in the experience in Japan and Chernobyl, in the type of radiation as well as the dose and dose rate—both higher in Japan—the results of studies among in utero- exposed survivors of the atomic bombings serve to provide some context. Head size at age 17 for those proximal to the bombing in Hiroshima (within 1500 m) was found to be 0.4–1.4 cm smaller compared with the more distal subjects, although individual doses were not estimated [14]. Otake and Schull [3] determined a 43.5% prevalence of small head size (smaller than 2 SD below the mean) among those exposed in utero to 1 Gy or more from the bombs in Hiroshima and Nagasaki, compared with a prevalence of 1.7% among the unexposed. Since small head size was defined as 2 or more SD less than the mean, and SD in most groups was between 1.5 and 1.9 cm [3], this suggests a reduction of between 3.0 – 3.8 (=2 × 1.5 – 2 × 1.9) cm in head circumference in this high dose group, who received a mean dose of 1.302 Gy. This would indicate a reduction of between 3.0 × (0.435 – 0.017)/1.302 = 0.96 cm Gy−1 and 3.8 × (0.435 – 0.017)/1.302 cm Gy−1 = 1.22 cm Gy−1 both figures close to our figure of 0.954 Gy−1 (Table 2). However, since doses are higher in the A-bomb cohort, there is a greater likelihood of head size <2 SD below the mean. Otake and Schull [3] measured head size between the ages of 9 and 19, when influences in postnatal life may have played a role, whereas we measured head size at birth.
Our findings of a significant decrease in chest circumference with increasing fetal I-131 thyroid dose (Table 3) can also be compared with those in the atomic bomb survivors exposed in utero. Nakashima [15] documented a change in chest circumference of −1.37 ± 0.53 cm/Gy, which is statistically compatible with the estimate we derive of −0.884 cm/Gy (95% CI −1.573, −0.103) (Table 3). As with our data, the effect was greatest for exposures earliest in gestation [15].
The dose-related reduction in neonatal length we observed, −0.577 cm/Gy (95% CI −1.392, 0.259) (Table 4) is in the same direction as, but less than, the estimates derived from the in utero-exposed Japanese atomic bomb survivors, of −2.65 ± 0.65 cm/Gy [15]. With respect to birth weight, in the full dataset our central estimate of change in birth weight with fetal dose, 42.700 g/Gy (95% CI −78.439, 162.354) (Table 5) is opposite in direction to the change in weight at ages 9–19 in the atomic bomb survivors, estimated as −2460 ± 740 g/Gy [15]. In the medical records subset (Table 10), the estimate is negative (−135.309 g/Gy (95% CI −275.303, 25.310, P = 0.075), although still less than the estimate of Nakashima [15]. In both cases our estimates have wide confidence intervals.
Our finding of a significant dose-related increase in gestational length is also notable but somewhat difficult to interpret. Interestingly, an earlier survey of mother–child pairs from contaminated regions of Belarus and Ukraine over the period from 1983 to 1990 [16] reported a 1.5-2-fold increase in post-term births after the accident, as well as some increase in macrosomia. The possible mechanism for radiation affecting the triggers for term delivery remains unclear.
Neonatal anthropometrics have also been studied in Belarus, in a cohort of 250 children exposed in utero to I-131 from the Chernobyl accident and a comparable group of 250 unexposed controls [17]. There were no significant differences (P ≥ 0.95) in head circumference, birth weight or neonatal length between the groups of ‘exposed’ and ‘unexposed’ children. Analyses were not carried out looking at varying levels of absorbed thyroid dose. Moreover, 55% of the ‘exposed’ children were 16 or more weeks of gestation at the time of the accident, and so were beyond the radiosensitive period. Finally, the statistical uncertainties given the small sample size and low power to detect differences imply that the results of the two studies are not inconsistent.
Head size is recognized as an important reflection of growth of the brain [18], and the fetal brain appears to be particularly sensitive to radiation early in gestation, during development of the central nervous system [19]. In early pregnancy the fetus is so small that there may be little difference in the I-131 dose to the thyroid and to the brain. At one month, the fetus is ~1 mm long and weighs ~1 g. At 2 months it is ~12 mm long and weighs ~4 g; and at 3 months it is ~47 mm long and weighs ~30 g. Beta rays from I-131 have energy mostly ~0.6 MeV and can travel 5 mm in water.
The mechanism(s) for an effect on head size from prenatal I-131 exposure is not entirely clear, but it may include maternal or fetal hypothyrodisim during critical periods of brain development, or interference with the HPT axis governing growth. In children with cancer receiving radiotherapy, the growth hormone axis has been found to be highly vulnerable to negative effects, with hypopituitarism following high dose cranial irradiation and precocious puberty in girls seen at lower therapeutic doses [20]. It is possible our finding may also be growth-related since the decrement in head circumference among those exposed early in gestation—a period corresponding to the highest velocity of head growth—is greater than the overall decrement associated with dose.
Potential limitations of the study should be considered. Head circumference at birth is influenced by the length of gestation and by gender of the offspring as well as by a variety of environmental, genetic and maternal factors, in particular maternal height and weight [21–24], parity [25], and cigarette smoking [26]. Although we are able to adjust for the confounding effects of many of these, including to a great extent maternal size, we lack information on certain others, such as smoking and alcohol consumption. While, there is no a priori reason to expect that lifestyle factors would be correlated with in utero I-131 dose, there is the possibility that such confounding does exist.
The impact of accident-related stress is an issue that should be considered. Perceived risk of Chernobyl-related illness among mothers evacuated from contaminated regions has been linked to higher levels of anxiety and depression [27]. However, there is no evidence that maternal stress per se has any impact on the head circumference of offspring, although there could potentially be indirect effects mediated through stress-related behaviors such as maternal malnutrition. Maternal stress has been reported to increase risk of preterm delivery [28], but not later delivery times as observed here.
Also, while we have individual estimates of prenatal thyroid dose from intakes of I-131 by the mother, clearly differentiated from any postnatal component, we do lack data on exposure to external radiation during pregnancy. However, doses for the settlements where cohort members resided are expected to be small, approximately 10 mGy on average [29], and hence are unlikely to have an appreciable impact on the results reported here.
A potential problem is the proportion of missing data, which for some endpoints, e.g., head circumference and chest circumference, is appreciable (Table 1). Absence of data was to a considerable extent due to the loss of medical records for cohort members from areas where the local medical facilities destroyed records after the mandatory 25-year retention period. Apart from this, there appears to be no systematic pattern to the missingness, as is also indicated by the striking similarity in the distribution of endpoints and covariates in the full cohort and in the subset with data taken from medical records (Table 1). Preliminary investigations using multiple imputation [30] and pattern-mixture modeling [31] to deal with the missing data issue suggest that it has not introduced a substantial bias.
Several points need to be borne in mind in interpreting the findings. We have presented the results as effect sizes at 1 Gy, while the mean dose in the analytic sample is far smaller—about 0.05–0.06 Gy (Table 1). Even at 1 Gy, the estimated reduction in head circumference of −1.0 cm represents a decrease of only about half a SD and is similar in magnitude to the 1 cm difference in head size at birth among U.S. males and females [32, 33]. Hence, while the finding is noteworthy given the Japanese data, there are likely to be only small changes in cognitive function or child development among exposed cohort members whose doses are generally lower (see Table 1). The effects on IQ and academic performance reported in Japan [34] were seen only in children with ‘small heads’—that is, with head circumference more than 2 SD below the mean—a definition sometimes used for diagnosing microcephaly (Centers for Disease Control and Prevention (CDC), 2016). Studies in individuals with more modest reductions in head size at birth, within the normal range, do not find that IQ or cognitive performance in childhood or adult life is substantially affected [35–38]. Rather, genetic factors and postnatal influences such as socioeconomic environment appear to be far stronger influences on cognitive function [39, 40]).
Our study has several strengths. It is based on a relatively large, well-established cohort of exposed mother–child pairs for whom individual prenatal thyroid I-131 doses have been estimated. It is virtually the first examination of neonatal outcomes and in utero exposure to I-131, the typical component of nuclear accident releases.
Conclusions
We have analyzed a range of informative endpoints, based largely on recorded measurements, and the reductions we observed in head size and chest circumference are compatible with those in the Japanese atomic bomb survivors exposed prenatally to external radiation, suggesting that the biological effects on the fetus of internal exposure to Iodine-131 are similar to those of penetrating external radiation. Further follow-up of the cohort would help to identify the long-term consequences of prenatal radiation exposure and assess the clinical significance of the results obtained.
Acknowledgements
Drs. Gilbert Beebe and Robert Miller of NCI first encouraged us to study individuals exposed in utero to radioactive fallout from Chernobyl. We thank Drs. Louise Brinton, Nori Nakamura and Fred Mettler for insightful comments. Oleksandr Zvinchuk and Oles Lapikura made valuable contributions to the ongoing work. The authors are grateful for the detailed and helpful comments of the referee. The study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics, and the Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Appendix
See Tables 7, 8, 9, 10 and 11.
Table 7.
Multivariate regression model to assess the relationship of head circumference (cm) with fetal dose, restricted to individuals with data taken from medical records, or medical records and logs
| Parameter estimate (+95% CI) | 2-sided P value | |
|---|---|---|
| Fetal dose (cm/Gy) | −0.954 (−1.493, −0.329) | 0.005 |
| Trimester 1 versus trimester 3 | −0.214 (−0.524, 0.054) | 0.134 |
| Trimester 2 versus trimester 3 | −0.188 (−0.488, 0.060) | 0.176 |
| Parity ≥1 versus parity 0 | 0.036 (−0.217, 0.267) | 0.771 |
| Gestation weeks (cm/week) | 0.307 (0.214, 0.413) | <0.001 |
| Baby sex female versus baby sex male | −0.307 (−0.543, −0.095) | 0.008 |
| Twin versus singleton | −1.190 (−2.709, −0.284) | 0.010a |
| Maternal age (cm/year) | 0.010 (−2.930, 2.793) | 0.936 |
| Maternal height (cm/cm) | 0.005 (−0.033, 3.470) | 1.000 |
| Maternal weight at 1st clinic (cm/kg) | 0.059 (0.031, 0.138) | 0.010 |
| Model with separate dose × trimester | ||
| Dose in trimester 1 (cm/Gy) | −7.072 (−28.770, 53.512) | 0.660 |
| Dose in trimester 2 (cm/Gy) | −1.504 (−2.326, −0.716) | 0.003 |
| Dose in trimester 3 (or missing) (cm/Gy) | −0.275 (−1.121, 0.867) | 0.541 |
| Linear quadratic model in dose | ||
| Linear dose (cm/Gy) | −1.163 (−2.561, 0.304) | 0.113 |
| Quadratic dose (cm/Gy2) | 0.206 (−0.936, 1.335) | 0.709 |
P value derived from BCA-confidence intervals via Normal distribution
All regressions evaluated using 4999 bootstrap resamples. Model adjusted for trimester of exposure, parity, gestation weeks, baby’s sex, singleton/twin birth, maternal age at delivery of offspring, maternal height and maternal weight at first clinic visit. The estimates for the constant term and for indicators of covariate missingness are not given. Analysis of n = 887 persons with non-missing data on head circumference and fetal dose, with data taken from medical records and/or logs
Table 8.
Multivariate regression model to assess the relationship of chest circumference (cm) with fetal dose, restricted to individuals with data taken from medical records, or medical records and logs
| Parameter estimate (+95% CI) | 2-sided P value | |
|---|---|---|
| Fetal dose (cm/Gy) | −0.884 (−1.573, −0.103) | 0.023 |
| Trimester 1 versus trimester 3 | −0.017 (−0.357, 0.289) | 0.921 |
| Trimester 2 versus trimester 3 | 0.017 (−0.294, 0.301) | 0.912 |
| Parity ≥1 versus parity 0 | 0.188 (−0.091, 0.459) | 0.178 |
| Gestation weeks (cm/week) | 0.258 (0.165, 0.365) | <0.001 |
| Baby sex female versus baby sex male | −0.360 (−0.602, −0.136) | 0.005 |
| Twin versus singleton | −1.457 (−2.943, −0.409) | 0.025 |
| Maternal age (cm/year) | 0.003 (−3.133, 3.661) | 0.999 |
| Maternal height (cm/cm) | 0.009 (−0.085, 3.254) | 1.000 |
| Maternal weight at 1st clinic (cm/kg) | 0.056 (0.024, 0.131) | 0.018 |
| Model with separate dose × trimester | ||
| Dose in trimester 1 (cm/Gy) | −10.627 (−29.577, 39.067) | 0.291 |
| Dose in trimester 2 (cm/Gy) | −1.592 (−2.310, −0.345) | 0.006 |
| Dose in trimester 3 (or missing) (cm/Gy) | −0.004 (−0.883, 1.212) | 0.994 |
| Linear quadratic model in dose | ||
| Linear dose (cm/Gy) | 0.337 (−1.294, 1.944) | 0.679 |
| Quadratic dose (cm/Gy2) | −1.205 (−2.621, 0.234) | 0.095 |
All regressions evaluated using 4999 bootstrap resamples. Model adjusted for trimester of exposure, parity, gestation weeks, baby’s sex, singleton/twin birth, maternal age at delivery of offspring, maternal height and maternal weight at first clinic visit. The estimates for the constant term and for indicators of covariate missingness are not given. Analysis of n = 882 persons with non-missing data on chest circumference and fetal dose, with data taken from medical records and/or logs
Table 9.
Multivariate regression model to assess the relationship of neonatal length (crown-heel length, cm) with fetal dose, restricted to individuals with data taken from medical records, or medical records and logs
| Parameter estimate (+95% CI) | 2-sided P value | |
|---|---|---|
| Fetal dose (cm/Gy) | −0.561 (−1.489, 0.524) | 0.253 |
| Trimester 1 versus trimester 3 | −0.283 (−0.652, 0.081) | 0.123 |
| Trimester 2 versus trimester 3 | −0.211 (−0.538, 0.111) | 0.196 |
| Parity ≥1 versus parity 0 | 0.337 (0.021, 0.666) | 0.038 |
| Gestation weeks (cm/week) | 0.611 (0.492, 0.797) | <0.001 |
| Baby sex female versus baby sex male | −0.958 (−1.255, −0.676) | <0.001 |
| Twin versus singleton | −1.692 (−2.840, −0.151) | 0.029 |
| Maternal age (cm/year) | 0.018 (−0.035, 10.115) | 1.000 |
| Maternal height (cm/cm) | 0.067 (−1.179, 1.894) | 0.998 |
| Maternal weight at 1st clinic (cm/kg) | 0.023 (−0.044, 0.058) | 0.353 |
| Model with separate dose × trimester | ||
| Dose in trimester 1 (cm/Gy) | −25.035 (−72.363, −3.464) | 0.045 |
| Dose in trimester 2 (cm/Gy) | 0.238 (−1.002, 2.276) | 0.767 |
| Dose in trimester 3 (or missing) (cm/Gy) | −1.485 (−3.751, 0.155) | 0.134 |
| Linear quadratic model in dose | ||
| Linear dose (cm/Gy) | −1.106 (−3.391, 1.298) | 0.345 |
| Quadratic dose (cm/Gy2) | 0.550 (−1.367, 2.833) | 0.575 |
All regressions evaluated using 4999 bootstrap resamples. Model adjusted for trimester of exposure, parity, gestation weeks, baby’s sex, singleton/twin birth, maternal age at delivery of offspring, maternal height and maternal weight at first clinic visit. The estimates for the constant term and for indicators of covariate missingness are not given. Analysis of n = 1088 persons with non-missing data on neonatal length and fetal dose, with data taken from medical records and/or logs
Table 10.
Multivariate regression model to assess the relationship of birthweight (g) with fetal dose, restricted to individuals with data taken from medical records, or medical records and logs
| Parameter estimate (+95% CI) | 2-sided P value | |
|---|---|---|
| Fetal dose (g/Gy) | −135.309 (−275.303, 25.310) | 0.075 |
| Trimester 1 versus trimester 3 | 28.182 (−37.779, 92.298) | 0.393 |
| Trimester 2 versus trimester 3 | −23.459 (−83.411, 40.937) | 0.439 |
| Parity ≥1 versus parity 0 | 120.698 (61.496, 179.927) | <0.001 |
| Gestation weeks (g/week) | 148.322 (127.578, 166.017) | <0.001 |
| Baby sex female versus baby sex male | −171.855 (−225.452, −121.588) | 0.002 |
| Twin versus singleton | −762.013 (−912.490, 73.368) | 0.080 |
| Maternal age (g/year) | 5.499 (−3.851, 2065.143) | 0.282 |
| Maternal height (g/cm) | 12.915 (4.882, 712.024) | 0.002a |
| Maternal weight at 1st clinic (g/kg) | 11.193 (5.898, 16.962) | <0.001 |
| Model with separate dose × trimester | ||
| Dose in trimester 1 (g/Gy) | −2869.097 (−16, 192.029, 5437.288) | 0.477 |
| Dose in trimester 2 (g/Gy) | −23.532 (−191.909, 241.571) | 0.823 |
| Dose in trimester 3 (or missing) (g/Gy) | −265.390 (−528.084, 12.830) | 0.061 |
| Linear quadratic model in dose | ||
| Linear dose (g/Gy) | −258.731 (−676.398, 116.034) | 0.196 |
| Quadratic dose (g/Gy2) | 124.899 (−191.511, 474.730) | 0.434 |
P value derived from BCA-confidence intervals via Normal distribution
All regressions evaluated using 4999 bootstrap resamples. Model adjusted for trimester of exposure, parity, gestation weeks, baby’s sex, singleton/twin birth, maternal age at delivery of offspring, maternal height and maternal weight at first clinic visit. The estimates for the constant term and for indicators of covariate missingness are not given. Analysis of n = 1106 persons with non-missing data on birthweight and fetal dose, with data taken from medical records and/or logs
Table 11.
Multivariate regression model to assess the relationship of gestational age at delivery (weeks) with fetal dose, restricted to individuals with data taken from medical records, or medical records and logs
| Parameter estimate (+95% CI) | 2-sided P value | |
|---|---|---|
| Fetal dose (weeks/Gy) | 0.787 (0.273, 1.426) | 0.014 |
| Trimester 1 versus trimester 3 | −0.105 (−0.366, 0.149) | 0.430 |
| Trimester 2 versus trimester 3 | −0.027 (−0.244, 0.198) | 0.802 |
| Parity ≥1 versus parity 0 | 0.175 (−0.034, 0.399) | 0.110 |
| Baby sex female versus baby sex male | 0.018 (−0.179, 0.211) | 0.855 |
| Twin versus singleton | −0.845 (−1.785, −0.090) | 0.046 |
| Maternal age (weeks/year) | −0.017 (−0.045, 0.012) | 0.281 |
| Maternal height (weeks/cm) | 0.009 (−0.011, 0.030) | 0.577 |
| Maternal weight at 1st clinic (weeks/kg) | 0.015 (−0.004, 0.031) | 0.075 |
| Model with separate dose × trimester | ||
| Dose in trimester 1 (weeks/Gy) | 15.440 (4.421, 91.846) | 0.566 |
| Dose in trimester 2 (weeks/Gy) | 0.298 (−0.524, 1.078) | 0.410 |
| Dose in trimester 3 (or missing) (weeks/Gy) | 1.330 (0.560, 2.710) | 0.036 |
| Linear quadratic model in dose | ||
| Linear dose (weeks/Gy) | 2.210 (0.595, 3.790) | 0.014 |
| Quadratic dose (weeks/Gy2) | −1.423 (−2.836, −0.109) | 0.028 |
All regressions evaluated using 4999 bootstrap resamples. Model adjusted for trimester of exposure, parity, baby’s sex, singleton/twin birth, maternal age at delivery of offspring, maternal height and maternal weight at first clinic visit. The estimates for the constant term and for indicators of covariate missingness are not given. Analysis of n = 1065 persons with non-missing data on gestational age and fetal dose, with data taken from medical records and/or logs
Footnotes
Conflict of interest The authors have no financial conflicts.
References
- 1.Gonzalez AJ, Akashi M, Boice JD Jr, et al. Radiological protection issues arising during and after the Fukushima nuclear reactor accident. J Radiol Prot. 2013;33(3):497–571. doi: 10.1088/0952-4746/33/3/497. [DOI] [PubMed] [Google Scholar]
- 2.International Commission on Radiological Protection. The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann ICRP. 2007;37(2–4):1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Otake M, Schull WJ. Radiation-related small head sizes among prenatally exposed A-bomb survivors. Int J Radiat Biol. 1993;63(2):255–70. [DOI] [PubMed] [Google Scholar]
- 4.Otake M, Schull WJ. Radiation-related brain damage and growth retardation among the prenatally exposed atomic bomb survivors. Int J Radiat Biol. 1998;74(2):159–71. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Otake M, Schull WJ. Changes in the pattern of growth in stature related to prenatal exposure to ionizing radiation. Int J Radiat Biol. 1999;75(11):1449–58. [DOI] [PubMed] [Google Scholar]
- 6.International Commission on Radiological Protection. Doses to the embryo and fetus from intakes of radionuclides by the mother. A report of The International Commission on Radiological Protection. Ann ICRP. 2001;31(1–3):19–515. [DOI] [PubMed] [Google Scholar]
- 7.Gorman CA. Radioiodine and pregnancy. Thyroid. 1999;9(7):721–6. doi: 10.1089/thy.1999.9.721. [DOI] [PubMed] [Google Scholar]
- 8.Sutow WW, Conard RA, Griffith KM. Growth status of children exposed to fallout radiation on Marshall Islands. Pediatrics. 1965;36(5):721–31. [PubMed] [Google Scholar]
- 9.Hatch M, Brenner A, Bogdanova T, et al. A screening study of thyroid cancer and other thyroid diseases among individuals exposed in utero to iodine-131 from Chernobyl fallout. J Clin Endocrinol Metab. 2009;94(3):899–906. doi: 10.1210/jc.2008-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Likhtarov I, Kovgan L, Chepurny M, et al. Estimation of the thyroid doses for ukrainian children exposed in utero after the chernobyl accident. Health Phys. 2011;100(6):583–93. doi: 10.1097/HP.0b013e3181ff391a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao CR. Linear statistical inference and its applications. 2nd ed. Singapore: Wiley; 2002. [Google Scholar]
- 12.Efron B Better bootstrap confidence intervals. J Am Statist Assoc. 1987;82(397):171–85. doi: 10.2307/2289144. [DOI] [Google Scholar]
- 13.Davison AC, Hinkley DV. Bootstrap methods and their application. Cambridge: Cambridge University Press; 1997. p. 1–592. [Google Scholar]
- 14.Wood JW, Johnson KG, Omori Y. In utero exposure to the Hiroshima atomic bomb. An evaluation of head size and mental retardation: twenty years later. Pediatrics. 1967;39(3):385–92. [PubMed] [Google Scholar]
- 15.Nakashima E Relationship of five anthropometric measurements at age 18 to radiation dose among atomic bomb survivors exposed in utero. Radiat Res. 1994;138(1):121–6. [PubMed] [Google Scholar]
- 16.Kulakov VI, Sokur TN, Volobuev AI, et al. Female reproductive function in areas affected by radiation after the Chernobyl power station accident. Environ Health Perspect. 1993;101(Suppl 2):117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igumnov S, Drozdovitch V. The intellectual development, mental and behavioural disorders in children from Belarus exposed in utero following the chernobyl accident. Eur Psychiatry. 2000;15(4):244–53. [DOI] [PubMed] [Google Scholar]
- 18.Geraedts EJ, van Dommelen P, Caliebe J, et al. Association between head circumference and body size. Horm Res Paediatr. 2011;75(3):213–9. doi: 10.1159/000321192. [DOI] [PubMed] [Google Scholar]
- 19.Streffer C Radiation effects of exposure during prenatal development. Radiologe. 1995;35(3):141–7. [PubMed] [Google Scholar]
- 20.Darzy KH, Shalet SM. Hypopituitarism following radiotherapy. Pituitary. 2009;12(1):40–50. doi: 10.1007/s11102-008-0088-4. [DOI] [PubMed] [Google Scholar]
- 21.Merchant KM, Villar J, Kestler E. Maternal height and newborn size relative to risk of intrapartum caesarean delivery and perinatal distress. BJOG. 2001;108(7):689–96. [DOI] [PubMed] [Google Scholar]
- 22.Bisai S Maternal height as an independent risk factor for neonatal size among adolescent bengalees in kolkata, India. Ethiop J Health Sci. 2010;20(3):153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Britto RP, Florencio TM, Benedito Silva AA, Sesso R, Cavalcante JC, Sawaya AL. Influence of maternal height and weight on low birth weight: a cross-sectional study in poor communities of northeastern Brazil. PLoS ONE. 2013;8(11):e80159. doi: 10.1371/journal.pone.0080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thame M, Osmond C, Trotman H. Fetal growth and birth size is associated with maternal anthropometry and body composition. Matern Child Nutr. 2015;11(4):574–82. doi: 10.1111/mcn.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ong KK, Preece MA, Emmett PM, Ahmed ML, Dunger DB, Team AS. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: longitudinal birth cohort study and analysis. Pediatr Res. 2002;52(6):863–7. doi: 10.1203/00006450-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Jaddoe VW, Verburg BO, de Ridder MA, et al. Maternal smoking and fetal growth characteristics in different periods of pregnancy: the generation R study. Am J Epidemiol. 2007;165(10):1207–15. doi: 10.1093/aje/kwm014. [DOI] [PubMed] [Google Scholar]
- 27.Bromet EJ, Gluzman S, Schwartz JE, Goldgaber D. Somatic symptoms in women 11 years after the Chornobyl accident: prevalence and risk factors. Environ Health Perspect. 2002;110(Suppl 4):625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz RJ, Fullerton J, Dudley DJ. The interrelationship of maternal stress, endocrine factors and inflammation on gestational length. Obstet Gynecol Surv. 2003;58(6):415–28. doi: 10.1097/01.OGX.0000071160.26072.DE. [DOI] [PubMed] [Google Scholar]
- 29.Bouville A, Likhtarev IA, Kovgan LN, Minenko VF, Shinkarev SM, Drozdovitch VV. Radiation dosimetry for highly contaminated Belarusian, Russian and Ukrainian populations, and for less contaminated populations in Europe. Health Phys. 2007;93(5):487–501. doi: 10.1097/01.HP.0000279019.23900.62. [DOI] [PubMed] [Google Scholar]
- 30.Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. p. 1–320. [Google Scholar]
- 31.Little RJA. Pattern-mixture models for multivariate incomplete data. J Am Statist Assoc. 1993;88(421):125–34. doi: 10.2307/2290705. [DOI] [Google Scholar]
- 32.Buck-Louis GM, Grewal J, Albert PS, et al. Racial/ethnic standards for fetal growth: the NICHD fetal growth studies. Am J Obstet Gynecol. 2015;213(4):449e1–41. doi: 10.1016/j.ajog.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007–2010. Vital Health Stat. 2012;11(252):1–48. [PubMed] [Google Scholar]
- 34.Schull WJ, Otake M. Cognitive function and prenatal exposure to ionizing radiation. Teratology. 1999;59(4):222–6. doi:. [DOI] [PubMed] [Google Scholar]
- 35.Broekman BF, Chan YH, Chong YS, et al. The influence of birth size on intelligence in healthy children. Pediatrics. 2009;123(6):e1011–6. doi: 10.1542/peds.2008-3344. [DOI] [PubMed] [Google Scholar]
- 36.Heinonen K, Raikkonen K, Pesonen AK, et al. Prenatal and postnatal growth and cognitive abilities at 56 months of age: a longitudinal study of infants born at term. Pediatrics. 2008;121(5):e1325–33. doi: 10.1542/peds.2007-1172. [DOI] [PubMed] [Google Scholar]
- 37.Gale CR, O’Callaghan FJ, Bredow M, Martyn CN. Avon Longitudinal Study of P, Children Study T. The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics. 2006;118(4):1486–92. doi: 10.1542/peds.2005-2629. [DOI] [PubMed] [Google Scholar]
- 38.Martyn CN, Gale CR, Sayer AA, Fall C. Growth in utero and cognitive function in adult life: follow up study of people born between 1920 and 1943. BMJ. 1996;312(7043):1393–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva A, Metha Z, O’Callaghan FJ. The relative effect of size at birth, postnatal growth and social factors on cognitive function in late childhood. Ann Epidemiol. 2006;16(6):469–76. doi: 10.1016/j.annepidem.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 40.Jefferis BJMH, Power C, Hertzman C. Birth weight, childhood socioeconomic environment, and cognitive development in the 1958 British birth cohort study. BMJ. 2002;325(7359):305. [DOI] [PMC free article] [PubMed] [Google Scholar]


