Abstract
Clinically stable patients with type 2 diabetes mellitus (T2DM) and coronary artery disease (CAD) are not often thought to present with the symptom of typical angina. We sought to enumerate the proportion of patients presenting with typical angina or other cardiac symptoms and to elucidate what important clinical variables are associated with the presence of typical angina in patients with T2DM and angiographically documented CAD. Symptoms of angina, anginal equivalents or an absence of symptoms were obtained utilizing baseline data from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial (n=2319). A bivariate analysis stratified by the presence or absence of prior revascularization and logistic regression modeling with a stepwise covariate selection was used. Our results noted that 82% of patients had symptoms while 18 % presented asymptomatically. This was further divided approximately into typical angina (1/5th); anginal equivalents (1/5th); combination (2/5th); asymptomatic (1/5th). A history of prior revascularization was a determinant of the type of symptom presentation in regards to the variables sex, age, current insulin use, myocardial jeopardy index score and use of beta-blockers. In the multivariable logistic regression analysis, of the available candidate variables only a history of beta-blocker use (OR=1.53, 95% CI 1.24–194, p<0.0001 and prior percutaneous coronary intervention (OR=1.55, 95% CI 1.24–1.94, p<0.0001) had a higher odds of an association with typical angina. In conclusion, a large proportion of patients with T2DM and CAD indeed have symptoms. Future studies of long term outcomes associated with these symptoms are needed.
Keywords: symptoms, chest pain, diabetes mellitus, coronary artery disease
Introduction
In order to gain an appreciation of symptom presentation in a select group of patients with type 2 diabetes mellitus (T2DM) and angiographically documented coronary artery disease (CAD) we sought to: a) enumerate the proportion of each symptom noted. b) elucidate the important demographic and clinical variables independently associated with the presence of typical angina pectoris. c) assess the association of fibrinolytic variables plasminogen activator inhibitor-1 (PAI-1) antigen, PAI-1 activity and tissue plasminogen activator (t-PA) to the symptom typical angina pectoris.
Methods
To meet the aims as noted above, a cross-sectional analysis was designed utilizing baseline data at the time of initial patient entry into the Bypass Angioplasty Revascularization Investigation 2 Diabetes trial (BARI 2D). The study design, patient characteristics and primary outcomes of the primary BARI 2D trial have been published1–4. In brief, the BARI 2D study was a multi-center National Institutes of Health trial that tested two simultaneous hypotheses in a 2×2 factorial design (immediate versus delayed or no revascularization and insulin sensitizing agents versus insulin providing agents) in patients with T2DM and angiographically documented stable CAD who all received optimal intensive medical therapy to control risk factors.
To obtain entry into the trial every patient had to have a coronary angiogram documenting ≥ 1 vessel with a ≥ 50% stenosis that was suitable for revascularization by either percutaneous coronary intervention (PCI) or by coronary artery bypass grafting surgery (CABG) with either objective documentation of ischemia or subjective documentation of angina with a ≥ 70% stenosis. Major exclusion criteria included a definite need for revascularization as judged by the attending cardiologist, left main coronary artery stenosis, planned intervention on a bypass graft, advanced congestive heart failure, elevated creatinine and poorly controlled diabetes mellitus (HgbA1c >13%).
Patients were questioned regarding their symptoms at the time of their initial baseline visit. The symptoms that were reported were within 6 weeks prior to this baseline visit. The symptoms reported were then categorized by clinical site staff as typical angina pectoris, anginal equivalent (shortness of breath, dyspnea on exertion, exertional fatigue, nausea, unexplained diaphoresis, other) or asymptomatic (no angina or anginal equivalents). Typical angina pectoris was defined as a discomfort or pain located in the chest or upper epigastrium described as a pressure, heaviness, tightness, squeezing, burning, or choking sensation that suggested an ischemic heart disease diagnosis. Patients that had symptoms of both typical angina pectoris and anginal equivalents were categorized as typical angina.
The fibrinolytic variables used were PAI-1 antigen, PAI-1 activity and t-PA. The biological basis for this addition was felt to be that elevated PAI-1 is likely to be associated with impaired fibrinolysis and promote a procoagulatory milieu that may contribute to acceleration of coronary artery disease with precipitation of events including angina or anginal equivalents. Tissue plasminogen activator is known to track with PAI-1. This tracking reflects a complexing of t-PA with PAI-1 with a consequent diminished clearance of complexed t-PA that is biochemically inactive.
Of the 2368 patients enrolled in the BARI 2D study, 2321 had 80% of their baseline data available for analysis. Of these, 2 patients did not report angina status within 6 weeks prior to randomization. This report includes the data from 2319 patients. Coronary revascularization was noted as the presence or absence of either PCI or CABG. An initial unadjusted and subsequent bivariate analysis stratified by revascularization method was performed. A p-value of < 0.05 was used to determine statistical significance. The p-values in the baseline table refer to Cochran-Mantel-Haenszel tests of general association among the three symptom groups after controlling for cardiac revascularization status. Differences among the symptoms groups for continuous variables were tested using ANOVA stratified by revascularization status. Multivariable stepwise logistic regression analysis was used to determine independent associations between baseline demographic, clinical and angiographic variables and typical angina. The model was built using stepwise regression of the candidate variables with a p-value of 0.1 to enter the model and 0.05 to be included (stay) in the model. The candidate variables were: age, sex, smoking status, exercise status, duration of diabetes mellitus, body mass index, systolic blood pressure, diastolic blood pressure, hemoglobin A1C level, LDL-cholesterol, HDL-cholesterol, total cholesterol, triglycerides, ankle-brachial index, history of lung disease, myocardial infarction, stroke, hypertension, non-coronary artery disease, PCI, stent, CABG, myocardial jeopardy index score5, coronary artery stenosis > 70%, current beta blocker, calcium channel blocker, statin, thiazolidinedione and insulin use. Left ventricular ejection fraction was not placed in the regression model due to the exclusion of advanced congestive heart failure patients in the main trial. Myocardial jeopardy index was defined as the percentage of left ventricular myocardium jeopardized by ≥ 50% stenosis5. Once the demographic and clinical variables having independent associations with typical angina were determined, baseline fibrinolysis measures of PAI-1 antigen, PAI-1 activity and t-PA were added to the final typical angina model to determine if they added any more information to the model. Since PAI-1 antigen and activity measures were skewed, they were transformed using the natural log. Two further similar logistic regression analyses were performed in patients with and without prior revascularization. SAS v.9.2 software was used for all statistical analysis.
Results
The distribution of symptoms reported were typical angina (n=437, 19%), anginal equivalents (n=493, 21%), combination of typical angina and anginal equivalents (n=971, 42%) and asymptomatic (n=418, 18%). Baseline left ventricular ejection fraction was 57.2 ± 10.9% (SD) in the entire cohort, 57.1 ± 11.0 % in the PCI stratum and 57.5 ± 10.8% in the CABG stratum. A history of prior coronary revascularization as well as the type of revascularization (PCI or CABG) was a determinant of symptom presentation (table 1). Regardless of prior revascularization status, males presented asymptomatically more often than females; females presented with typical angina more often; and older individuals presented less often with typical angina. The variations across symptoms stratified by revascularization in the baseline characteristics are noted in table 2. As noted in table 3, all three fibrinolytic variables (PAI-1 activity, PAI-1 antigen and t-PA) varied significantly across the groups (p=0.0071, p=0.0058, p=0.0003 respectively) after adjustment for prior revascularization status.
Table 1.
Symptom presentation by the presence and type of prior coronary revascularization. PCI=percutaneous coronary intervention, CABG=coronary artery bypass grafting. Nominal p-values shown in the table refer to global tests of equality among symptom groups.
| Variable | Total | Typical Angina | Anginal Equivalent | Asymptomatic | p-value |
|---|---|---|---|---|---|
|
| |||||
| (N= 2319) | (N=1408) | (N=493) | (N=418) | ||
| Any prior coronary revascularization | 23.5% | 26.2% | 22.1% | 16.0% | <0.0001 |
| Prior PCI | 19.6% | 22.2% | 17.2% | 13.4% | <0.0001 |
| Prior CABG | 6.4% | 7.0% | 6.9% | 4.1% | 0.09 |
Table 2.
A comparison of baseline demographics, clinical, laboratory and angiographic variables shown by symptom presentation stratified by presence or absence of prior coronary revascularization. SD= standard deviation, DM =diabetes mellitus, TZD= Thiazolidinedione, Lung disease=chronic obstructive pulmonary disease & asthma, HbA1c = glycated hemoglobin, LDLc=low density lipoprotein cholesterol. Nominal p-values shown in the table refer to global tests of equality among symptom groups.
| No History of Prior Revascularization | History of Prior Revascularization | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Characteristic | Typical Angina (N=1039) | Anginal Equivalent (N=384) | Asymptomatic (N=351) | Typical Angina (N=369) | Anginal Equivalent (N=109) | Asymptomatic (N=67) | p-value |
|
| |||||||
| Male | 66.6% | 71.1% | 78.6% | 71.0% | 71.6% | 79.1% | <.0001 |
| Age at study entry, mean, SD (years) | 61.6, 8.9 | 63.1, 8.7 | 63.1, 8.9 | 61.8, 8.8 | 64.6, 9.6 | 65.5, 7.3 | <.0001 |
| Current smoker | 11.9% | 14.9% | 11.7% | 14.1% | 7.3% | 6.0% | 0.49 |
| Regular exercise | 22.9% | 24.0% | 34.2% | 25.0% | 23.9% | 43.3% | <.0001 |
| Age at DM diagnosis, mean, SD (years) | 51.0, 10.9 | 51.7, 10.9 | 52.7, 11.3 | 50.6, 10.9 | 52.1, 11.3 | 54.6, 8.6 | 0.094 |
| Duration of DM, mean, SD (years) | 10.2, 8.4 | 10.9, 9.1 | 9.8, 8.6 | 10.8, 9.2 | 11.8, 8.3 | 10.4, 7.6 | 0.2036 |
| Currently taking insulin | 26.9% | 30.7% | 21.1% | 33.1% | 31.2% | 25.4% | 0.0092 |
| Currently taking TZD | 15.0% | 21.6% | 21.4% | 18.2% | 28.4% | 34.3% | <0.0001 |
| HbA1c %, mean, SD | 7.68, 1.61 | 7.65, 1.61 | 7.57, 1.68 | 7.71, 1.62 | 7.74, 1.62 | 7.54, 1.38 | 0.19 |
| History of lung disease | 10.6% | 11.2% | 5.1% | 13.6% | 12.0% | 9.0% | 0.0043 |
| Sitting systolic blood pressure, mean, SD (mmHg) | 132.2, 20.7 | 132.9, 20.6 | 131.9, 19.6 | 129.2, 19.2 | 129.8, 16.3 | 134.2, 17.8 | 0.74 |
| Myocardial Jeopardy score, mean, SD (%) | 46.9, 24.2 | 42.9, 25.1 | 48.2, 24.1 | 38.3, 22.1 | 36.8, 24.5 | 44.4, 23.7 | 0.0015 |
| Coronary Stenosis > 70% | 62.6% | 59.6% | 66.9% | 64.0% | 73.1% | 67.2% | 0.25 |
| LDL-c (mg/dl) median, Q1-Q3 | 92.0 (72.0–116.0) | 96.0 (77.0–118.0) | 90.5 (74.0–111.0) | 89.0 (72.0–109.0) | 92.0 (69.0–115.0) | 93.6 (80.0–113.0) | 0.45 |
| Beta blocker | 74.7% | 64.8% | 64.4% | 82.3% | 74.3% | 80.6% | <.0001 |
| Calcium Channel Blocker | 30.3% | 30.5% | 28.2% | 37.0% | 35.8% | 32.8% | 0.63 |
| Any Nitrates | 55.3% | 25.8% | 27.1% | 63.6% | 45.9% | 32.8% | <.0001 |
| Statin | 71.8% | 71.0% | 68.9% | 85.9% | 86.2% | 92.5% | 0.88 |
Table 3.
Fibrinolytic variables shown by symptom presentation stratified by presence or absence of prior revascularization status. PAI-1 = plasminogen activator inhibitor, t-PA= tissue plasminogen activator. Nominal p-values shown in the table refer to global tests of equality among symptom groups.
| No history of prior revascularization | History of prior revascularization | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Variable | Typical Angina (N=1039) | Anginal Equivalent (N=384) | Asymptomatic (N=351) | Typical Angina (N=369) | Anginal Equivalent (N=109) | Asymptomatic (N=67) | p-value |
|
| |||||||
| PAI-1 Activity (AU/ml) median, Q1- Q3 | 16 10.0 – 26.0 | 18 11.0 – 28.0 | 15 9.3 – 25.0 | 17 9.9–28.0 | 13 9.0–23.0 | 17 9.0 – 27.0 | 0.007 |
| PAI-1 Antigen (ng/ml) median, Q1-Q3 | 23 16.0 – 35.0 | 24 16.0 – 36.0 | 22 14.0 – 33.0 | 24 15.0 – 36.0 | 21.5 13.0 – 33.0 | 23 14.0 – 35.0 | 0.006 |
| t-PA(ng/ml) mean, SD | 10.36, 4.05 | 10.49, 3.97 | 9.69, 3.71 | 10.29, 4.52 | 9.59, 3.88 | 9.56, 3.47 | 0.0003 |
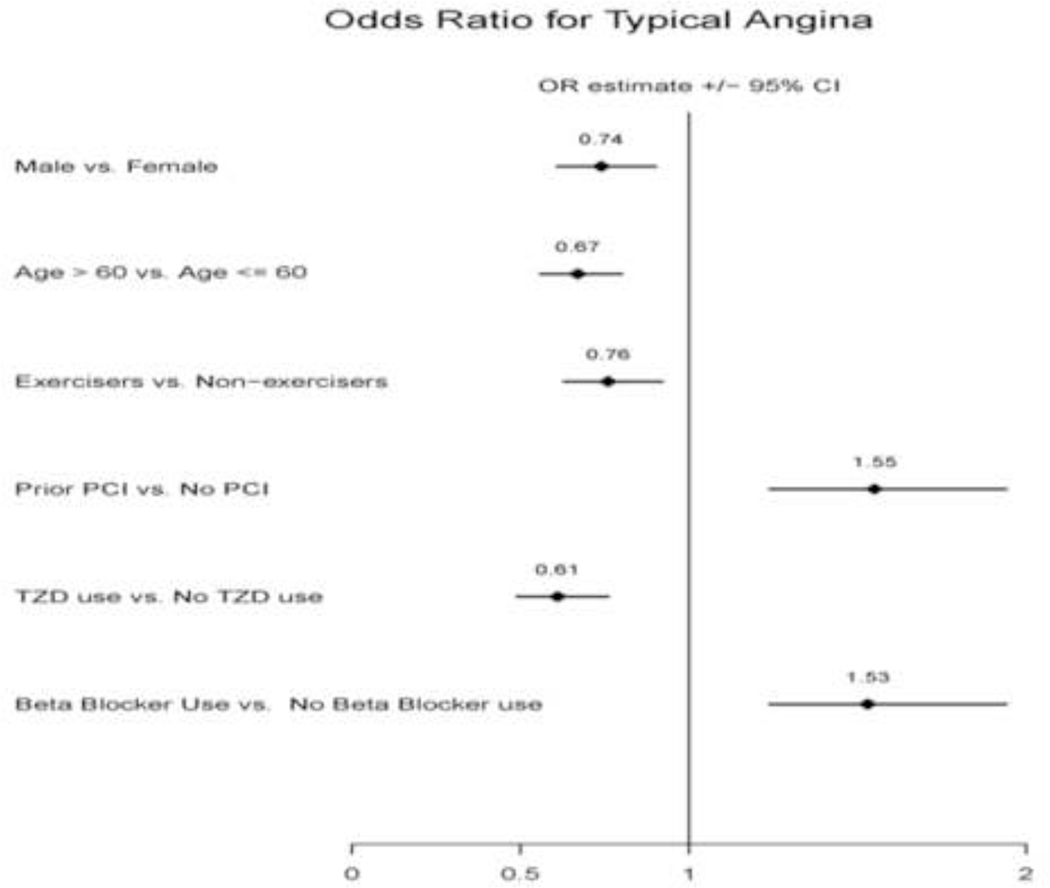
In the multivariable logistic regression analysis utilizing the entire cohort, only participants with a history of beta-blocker use (OR=1.53, 95% CI 1.24–1.94, p<0.0001) and prior percutaneous coronary intervention (OR=1.55, 95% CI 1.24–1.94, p<0.0001) were more likely to report typical angina. Participants that were male gender, older than 60 years, current exercisers, and had a history of thiazolidinedione use were less likely to report typical angina (Figure 1). PAI-1 antigen and PAI-1 activity were not significantly associated (p> 0.08) with typical angina. However, higher t-PA levels were independently associated with higher odds of typical angina (OR 1.03 for every one unit of increase in t-PA level, 95% CI 1.01 –1.06, p=0.008). In table 4, the adjusted covariates that were statistically significant are shown stratified by presence or absence of prior revascularization. In patients with prior revascularization, PAI-1 antigen, PAI-1 activity and t-PA were not significantly associated with typical angina. In those who did not undergo prior revascularization, t-PA continued to be independently associated with a higher odds of typical angina (OR 1.03, 1.01 –1.06, p=0.02).
Figure 1.
Multivariable logistic regression analysis showing independent adjusted odds ratios for typical angina within 6 weeks prior to the baseline visit in the BARI 2D clinical trial (n=2311 for final model). Age in years, PCI = percutaneous coronary intervention, TZD= thiazolidinedione. The diamond represents the odds ratio estimate and the line shows the 95% CI.
Table 4.
Independent adjusted odds ratio of covariates in patients with and without a history of prior revascularization in a multivariable logistic regression analysis for typical angina within 6 weeks prior to the baseline visit in the BARI 2D clinical trial. TZD= thiazolidinedione.
| No history of prior revascularization (N=1748) | History of prior revascularization (n=544) | |||
|---|---|---|---|---|
|
| ||||
| Variable | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value |
|
| ||||
| Male | 0.70 (0.57 – 0.87) | <0.002 | ||
| Age >60 years | 0.68 (0.55 – 0.83) | <0.0001 | 0.54 (0.36 – 0.72) | 0.002 |
| Regular Exercise | 0.77 (0.61 – 0.96) | 0.018 | ||
| History of hypertension | 1.43 (1.11 – 1.83) | 0.006 | ||
| Use of TZD | 0.65 (0.51 – 0.84) | 0.001 | 0.52 (0.34 –0.79) | 0.002 |
| Beta-blocker use | 1.56 (1.26 – 1.93) | <0.0001 | ||
Discussion
This analysis brings to the forefront a few important points which will be individually dealt with in the following paragraphs. First, a large proportion of patients with T2DM and documented stable CAD do indeed have symptoms. Second, prior coronary revascularization in general and particularly the type of prior revascularization, is associated with the type of symptom presentation. Third, future studies will need to assess the prognostic implications of typical angina or dyspnea6 (or other anginal equivalents) in this or a similar cohort which was not addressed in the current baseline analysis.
We were able to demonstrate that in a population of patients with T2DM and angiographically proven CAD, a large proportion (82%) have symptoms; only 18 % had no symptoms. Symptom status in the present study can be divided approximately into typical angina (1/5th); anginal equivalents (1/5th); combination of typical angina and anginal equivalents (2/5th); no angina or anginal equivalents (1/5th). Typical angina pectoris as the sole symptom was noted in 19% in our study compared with 28% of diabetic patients in a very early study utilizing thallium scintigraphy. The variations between the two studies are probably a result of different study designs7.
We felt that the type of symptoms that patients present with may vary with whether they have had prior revascularization or not and also with the type of prior coronary revascularization i.e., PCI or CABG. A paucity of data exists on this topic. In the current study typical angina was noted more often (26.2%, p<0.0001) compared to the other symptoms in patients with a history of any prior coronary revascularization. Comparing the two revascularization techniques, typical angina was noted in 22.0 % (p<0.0001) of patients with a history of prior PCI but only 7.0% (p=0.09) in those who had prior CABG suggesting variations in symptom presentation by mode of revascularization. This was validated in the initial multivariable logistic regression analysis when participants with a history of percutaneous coronary intervention had 55% higher odds of reporting typical angina (p<0.0001). Slight variations in the covariates that were significantly associated with typical angina were also noted between those who had prior revascularization and those who did not have prior revascularization. Further work needs to be done to explore the interaction between the type of prior revascularization and symptom presentation using multivariable analysis techniques on a hard outcome such as all-cause mortality.
Dyspnea is a well established symptom of cardiorespiratory illness and is usually “applied to sensations experienced by individuals who complain of unpleasant or uncomfortable respiratory sensations”8. The etiology of dyspnea in cardiac diseases is quite often related to the underlying left ventricular function9–10. In the present study, overall left ventricular ejection fraction was > 45% so it is less likely that the shortness of breath is related to underlying left ventricular systolic dysfunction. Thiazolidinedione use in patients with T2DM has been reported to cause or exacerbate edema in 2.5%–16.2% of patients and approximately 0.25–0.45%/year may experience symptoms of volume overload or heart failure11–12. Based on our analysis, thiazolidinedione use was associated with 39% lower odds of presenting with typical angina (p<0.0001) i.e., more likely presenting with anginal equivalents or asymptomatically. Dyspnea can also be a manifestation of pulmonary disease. However, only 4–6 % of the total cohort in this study had a diagnosis of chronic obstructive pulmonary disease or asthma and this covariate was not statistically significant in the multivariable analysis. Therefore, dyspnea in this study probably represents manifestations of varying degrees of obstructive coronary artery disease primarily or through a combination of other mechanisms leading to decreased left ventricular compliance (i.e., diastolic dysfunction) that was not evaluated in the present study.
The overall prognosis in patients with T2DM and CAD is thought to vary by the type of symptom presentation. Prior studies have suggested a poorer prognosis in patients presenting with dyspnea6, 13. Zellweger et al13, using a Cox proportional hazards model noted an adjusted hazard ratio of 2.1, 95% CI 1.24–3.42, (p=0.005) in patients with shortness of breath for the composite outcome of myocardial infarction or cardiac death. They also noted that in patients with shortness of breath event rates were significantly greater in those with abnormal myocardial perfusion SPECT imaging (13.2%), compared to normal myocardial perfusion SPECT imaging (3.3%), p=0.001. In a very well done epidemiological study Abidov et al6, described the annual mortality rate based on symptom presentation and the presence or absence of coronary artery disease. It was noted that in patients with no known coronary artery disease presenting with dyspnea the annual all-cause mortality rate was 6.2 %/year compared with typical angina, atypical angina, nonanginal chest pain or asymptomatic (p<0.001 for the difference across the other four groups.). In those patients with known coronary artery disease and presenting with dyspnea, the annual all-cause mortality rate was even higher at 11.7%/year (p<0.001 for the difference across the other four groups.). This suggests that the assessment of symptoms is of paramount importance for future prognostic implications. The present study examined data at baseline from the BARI 2D clinical trial. Further studies from this cohort will need to be undertaken to assess prognosis based on the presence or absence of typical angina or anginal equivalents.
A final intriguing question is whether underlying fibrinolytic biomarkers are associated with symptom development in patients with T2DM and stable CAD? Our initial analysis noted that values of fibrinolytic factors varied significantly with symptom presentation. However, in the multivariable model, only t-PA was significantly associated with typical angina in the entire cohort as well as in those who had no prior revascularization. Although, t-PA has been noted to predict future cardiovascular events in patients with established CAD14–15 there have been no prior studies that have assessed symptom presentation with underlying fibrinolytic activity. Further work is needed to better understand how symptoms correlate with the underlying fibrinolytic status16.
The limitation of this study is that it is cross-sectional in nature which reveals certain associations of interest but do not establish causal relationships. Also, the BARI 2D patient cohort is a highly select group of patients chosen on very strict inclusion and exclusion criteria. Therefore, due to entry bias our results may not be representative of those in the general patient population with CAD and T2DM. An important strength is that symptom ascertainment in a cohort of patients with T2DM and documented CAD have not been established and this study thus adds to the literature in this aspect.
Funding:
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) is funded by the National Heart, Lung and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, Nos. U01 HL061744, U01 HL061746, U01 HL061748, and U01 HL063804. Significant supplemental funding is provided by GlaxoSmithKline, Collegeville, PA, Bristol-Myers Squibb Medical Imaging, Inc., North Billerica, MA, Astellas Pharma US, Inc., Deerfield, IL, Merck & Co., Inc., Whitehouse Station, NJ, Abbott Laboratories, Inc., Abbott Park, IL, and Pfizer, Inc, New York, NY. Generous support is given by Abbott Laboratories Ltd., MediSense Products, Mississauga, Canada, Bayer Diagnostics, Tarrytown, NY, Becton, Dickinson and Company, Franklin Lakes, NJ, J. R. Carlson Labs, Arlington Hts., IL, Centocor, Inc., Malvern, PA, Eli Lilly and Company, Indianapolis, IN, LipoScience, Inc., Raleigh, NC, Merck Sante, Lyon, France, Novartis Pharmaceuticals Corporation, East Hanover, NJ, and Novo Nordisk, Inc. Princeton, NJ.
As an NIH funded trial, we are required to abide by the NIH PubMed Central Policy that we retain the right to provide a copy of the final manuscript to the NIH upon acceptance for publication by your journal, for public archiving in PubMed Central as soon as possible, but no later than 12 months after publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute, the National Institute of Diabetes And Digestive And Kidney Diseases, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production processerrorsmaybediscoveredwhichcouldaffectthecontent,andalllegaldisclaimers that apply to the journal pertain.
References:
- 1.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;24:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baseline characteristics of patients with diabetes and coronary artery disease enrolled in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Am Heart J 2008;3:528–53636 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magee MF, Isley WL. Rationale, design, and methods for glycemic control in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol 2006;97:20G–30G. [DOI] [PubMed] [Google Scholar]
- 4.Barsness GW, Gersh BJ, Brooks MM, Frye RL. Rationale for the revascularization arm of the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol 2006;97:31G–40G. [DOI] [PubMed] [Google Scholar]
- 5.Alderman EL SM. The angiographic definitions of the Bypass Angioplasty Revascularization Investigation. Coronary Artery Disease 1992;3:1189–1207 [Google Scholar]
- 6.Abidov A, Rozanski A, Hachamovitch R, Hayes SW, Aboul-Enein F, Cohen I, Friedman JD, Germano G, Berman DS. Prognostic significance of dyspnea in patients referred for cardiac stress testing. N Engl J Med 200518:1889–1898. [DOI] [PubMed] [Google Scholar]
- 7.Nesto RW, Phillips RT, Kett KG, Hill T, Perper E, Young E, Leland OS, Jr. Angina and exertional myocardial ischemia in diabetic and nondiabetic patients: assessment by exercise thallium scintigraphy. Ann Intern Med 1988;2:170–175. [DOI] [PubMed] [Google Scholar]
- 8.Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med 1999;1:321–340. [DOI] [PubMed] [Google Scholar]
- 9.ADHERE Scientific Advisory Committee:Acute Decompensated Heart Failure National Registry (ADHERE©) Core Module Q1 2006 Final Cumulative National Benchmark Report.: Scios, Inc. July 2006. [Google Scholar]
- 10.O’Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT-HF registry. J Card Fail 2005;3:200–205. [DOI] [PubMed] [Google Scholar]
- 11.Thiazolidinediones Yki-Jarvinen H.. N Engl J Med 2004;11:1106–1118. [DOI] [PubMed] [Google Scholar]
- 12.Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003;23:2941–2948. [DOI] [PubMed] [Google Scholar]
- 13.Zellweger MJ, Hachamovitch R, Kang X, Hayes SW, Friedman JD, Germano G, Pfisterer ME, Berman DS. Prognostic relevance of symptoms versus objective evidence of coronary artery disease in diabetic patients. Eur Heart J 2004;7:543–550. [DOI] [PubMed] [Google Scholar]
- 14.Gorog DA. Prognostic value of plasma fibrinolysis activation markers in cardiovascular disease. J Am Coll Cardiol 2010;24:2701–2709. [DOI] [PubMed] [Google Scholar]
- 15.Smith FB, Fowkes FG, Rumley A, Lee AJ, Lowe GD, Hau CM. Tissue plasminogen activator and leucocyte elastase as predictors of cardiovascular events in subjects with angina pectoris: Edinburgh Artery Study. Eur Heart J 2000;19:1607–1613. [DOI] [PubMed] [Google Scholar]
- 16.Sobel BE. Ancillary studies in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial: Synergies and opportunities. Am J Cardiol 2006;12A:53G–8G. [DOI] [PubMed] [Google Scholar]