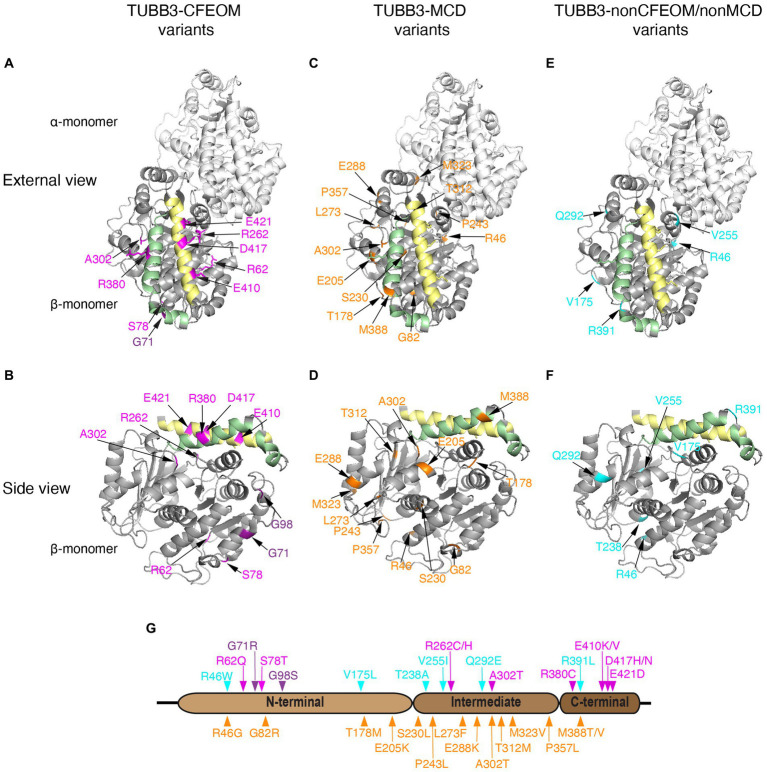
Figure 4.
Human TUBB3 amino acid substitutions. (A–F) Three-dimensional schematics of TUBB3 structure with locations of reported TUBB3 human substitutions highlighted generated using PyMol (https://www.rcsb.org/structure/1JFF) and rotated to reveal: ‘external views’ (row 1; A,C,E displayed as heterodimers with α-tubulin) and ‘side views’ (row 2: B,D,F, displayed as β-tubulin monomers) highlighting helices H11 (green) and H12 (yellow) on the outside of the cylindrical hollow microtubule. TUBB3-CFEOM substitutions are highlighted in magenta and TUBB3-CFEOM/MCD in purple (A,B). These substitutions are most often found in the C-terminal domain on or adjacent to helix H12 (yellow) on residues where motor protein interact. The exceptions are: R380 on helix H11 (green) and A302 that may interact with H11; R62 located in a loop mediating lateral interactions; and S78 together with TUBB3-CFEOM/MCD substitutions at G71 and G98 which cluster together and away from other variants near the E site of the GTP binding pocket. TUBB3-MCD substitutions are highlighted in orange (C,D). These substitutions are located primarily in regions that regulate GTP binding, heterodimer stability, and longitudinal and lateral interactions and away from variants in (A,B). Exceptions are A302 which is altered to a different residue in TUBB3-CFEOM and is located within a loop that could be important for both heterodimer stability and MAP/motor protein interactions. Similarly, M388 could regulate MAP/motor protein interactions, and is in proximity to residues at the plus-end of β-tubulin that mediate inter heterodimer contacts. TUBB3-nonCFEOM/nonMCD substitutions are highlighted in turquois (E,F). These substitutions are also removed from H11/H12 helices and are located in regions that regulate heterodimer stability and longitudinal and lateral interactions. Refer to Tischfield et al. (2011) for 3D domain schematics. (G) Two-dimensional schematic of TUBB3 N-terminal, intermediate, and C-terminal domains with human amino acid substitutions indicated. TUBB3-CFEOM (magenta), TUBB3-CFEOM/MCD (purple), and TUBB3-nonCFEOM/nonMCD (cyan) amino acid substitutions are shown above, while TUBB3-MCD amino acid substitutions (orange) are shown below the TUBB3 protein schematic.