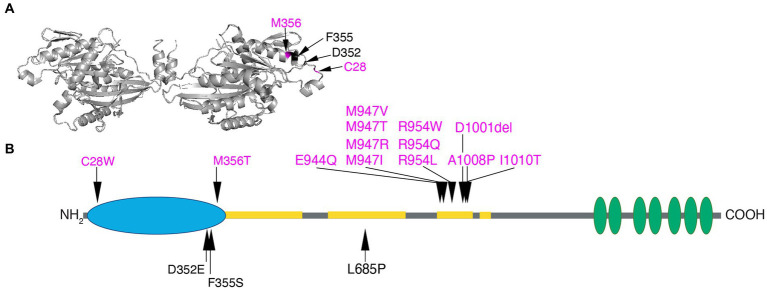
Figure 5.
Human KIF21A amino acid substitutions. (A) Three-dimensional schematic of the D. melanogaster kinesin-1 motor domain dimer (https://www.rcsb.org/structure/2Y5W) with the four human variants that alter residues in the motor domain mapped to the monomer on the right. C28 and M345 that cause isolated CFEOM are denoted in magenta and D352 and F355 that cause more syndromic CFEOM are denoted in black. The variants cluster on the lateral region of the motor domain removed from the ATP and microtubule binding sites. Data supports this as the site of the motor-third coiled-coil stalk domain interaction for KIF21A autoinhibition. (B) Two-dimensional schematic of KIF21A motor (blue), stalk (gray) and WD40 (green) domains. The coiled-coil regions of the stalk are denoted in yellow. Substitutions that cause isolated CFEOM are denoted in magenta above the KIF21A schematic. In addition to the two motor substitutions, note the clustering of KIF21A-CFEOM substitutions in the third coiled-coil region of the stalk. This region has been demonstrated to interact with the lateral aspect of the motor domain. The three substitutions that cause more syndromic CFEOM are noted in black below the KIF21A schematic. In addition to the two in the distal motor domain, L685P maps to the second coiled-coil and results in a syndromic phenotype similar to the E410K TUBB3 syndrome.