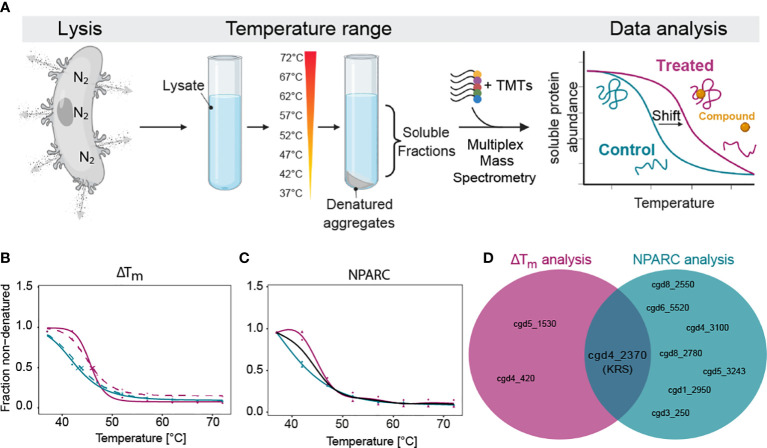
Figure 1.
Thermal proteome profiling (TPP) determines that CpKRS is the target of DDD01510706. (A) After excystation, Cryptosporidium sporozoites (wild type) were lysed using nitrogen cavitation. Lysate was incubated with or without DDD01510706 (10x EC50, 25 µM), aliquoted and subjected to a temperature range. Soluble proteins were precipitated and labelled with Tandem-Mass-Tags (TMTs), multiplexed, and analysed by mass spectrometry. Binding of a compound (yellow circle) to its protein target (correctly folded protein, magenta) commonly increases its thermal stability, often resulting in a measurable shift in the melting temperature (Tm) of the target protein. (B, C) In the presence of DDD01510706, KRS (magenta) demonstrated a statistically relevant increase in thermal stability (+2.5°C) compared to control samples (teal) as determined by (B) ΔTm analysis and (C) Non-Parametric Analysis of Response Curves (NPARC). (D) KRS is the only target identified by both analytic methods. Data represents 2 biological replicates with 1 technical replicate performed at each temperature. For ΔTm analysis, solid and dashed line represent the two biological replicates. For NPARC analysis, solid line represents null model and colored lines represented experimental samples. Created with BioRender.com.