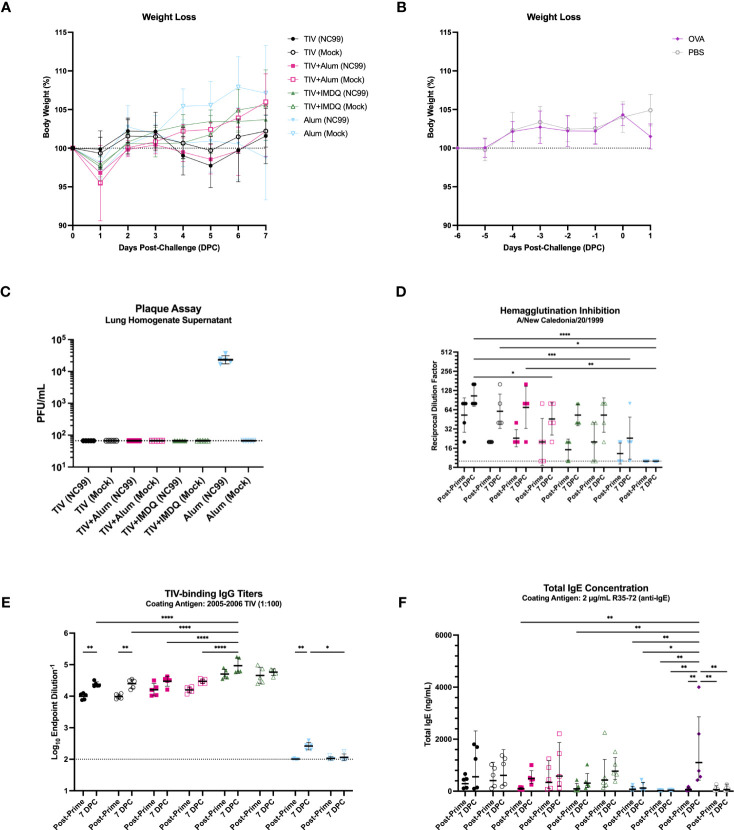
Figure 4.
TIV-vaccinated mice are protected against severe morbidity and viral replication with intact serological responses. (A) Weight loss of TIV, TIV+Alum, TIV+IMDQ, and Alum-vaccinated mice through 7 DPC following intranasal, sublethal (0.2 LD50), vaccine-matched influenza challenge or allantoic fluid mock-challenge. (B) Weight loss of OVA-sensitized and PBS control mice through 1 DPC following intranasal OVA challenge or no challenge, respectively. Line represents the average of 5 mice. (C) Lung viral titers from clarified lung left lobe homogenate supernatants. Line denotes limit of detection (66.67 PFU/mL). (D) Serum hemagglutination inhibition titers against NC99. Line denotes limit of detection (titer of 10). (E) Serum TIV-binding IgG titers. Line demotes limit of detection (titer of 100). (F) Total IgE concentration in serum. Bars denote the (C, D, F) geometric mean ± geometric standard deviation or (E) mean ± standard deviation. Statistical significance in (D–F) was determined via two-way ANOVA with Tukey’s multiple comparisons test: ****P < 0.0001, ***P = 0.0001 to 0.001, **P = 0.001 to 0.01, *P = 0.01 to 0.05.