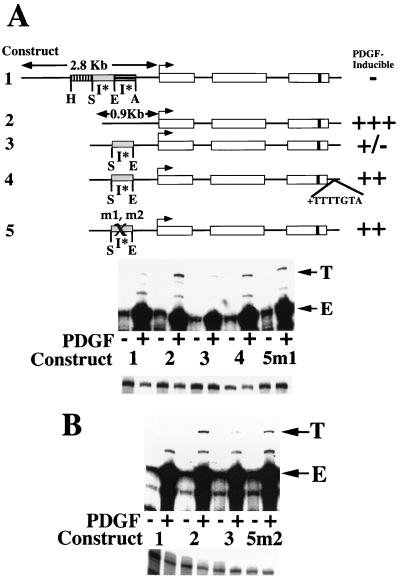
FIG. 4.
Necessity of 12-nt (5′) and 8-nt (3′) footprinted sequences for inhibition of MCP-1 induction. (A) At the top is a schematic of the structures of five tagged MCP-1 reporter constructs. Constructs 1 through 3 and 5 contain 104 bp of 3′ untranslated sequences (that include the polyadenylation signal but not the heptamer). Only construct 4 contains the heptamer within its 3′ untranslated sequences. The details of the MCP-1 schematics are otherwise as described in the legend to Fig. 1. Construct 2 is derived from construct 1 by removal of the 1,936-bp AocI-HincII 5′ fragment. Construct 3 is derived from construct 2 by readdition of the 695-bp SpeI-EcoRI fragment. Construct 4 is derived from construct 3 by addition of the heptamer TTTTGTA to the 3′ untranslated sequences. Constructs 5m1 and m2 are derived from construct 3 by site-directed mutation of nonoverlapping 12-nt (5′ subregion) and 8-nt (3′ subregion) portions, respectively, of the footprinted region shown in Fig. 3 (i.e., mutating the 12-base sequence GCCCCACCCCCA to ATGAAGTTGATC and the 8-base sequence GTCACCTG to TGGCTAGT within the otherwise unaltered 695-bp SpeI-EcoRI fragment). The PDGF-regulated 5′ inhibitory I* elements are indicated. The PDGF inducibilities of the constructs in transfection experiments are summarized on the right. At the middle are RNase protection assays of 40 μg of total cellular RNA that was prepared from NIH 3T3 fibroblasts transiently transfected with 5 μg of the constructs shown, allowed to become quiescent, and then not exposed (−) or exposed (+) to the B-B isoform of PDGF (30 ng/ml) for 3 h. The numbers refer to the tagged MCP-1 constructs described at the top. The 305- and 241-nt protected fragments corresponding to expression of the transfected and tagged (T) and endogenous (E) MCP-1 genes, respectively, are indicated. The experiment was performed four times with similar results. The PDGF inductions obtained with construct 5m1 were 1.6 to 5.2 times greater than those obtained with construct 3 in these transfections. The PDGF induction increases observed with construct 5m1, compared to construct 3, are statistically significant (P < 0.05 by the Wilcoxon two-sample test). At the bottom are RNase protection assays of 15 μg of total cellular RNA taken from the transfections shown above and analyzed with an alpha-globin riboprobe. (B) At the top are RNase protection assays of 40 μg of total cellular RNA prepared from NIH 3T3 fibroblasts transiently transfected with 4 μg of the constructs shown, allowed to become quiescent, and then not exposed (−) or exposed (+) to the B-B isoform of PDGF (30 ng/ml) for 3 h. The numbers refer to the tagged MCP-1 constructs described at the top. The 305- and 241-nt protected fragments corresponding to expression of the transfected and tagged (T) and endogenous (E) MCP-1 genes, respectively, are indicated. The experiment was performed four times with similar results. The PDGF inductions obtained with construct 5m2 were 1.8 to 2.7 times greater than those obtained with construct 3 in these transfections. The PDGF induction increases observed with construct 5m2, compared to construct 3, are statistically significant (P < 0.05 by the Wilcoxon two-sample test). At the bottom are RNase protection assays of 15 μg of total cellular RNA taken from the transfections shown above and analyzed with an alpha-globin riboprobe.