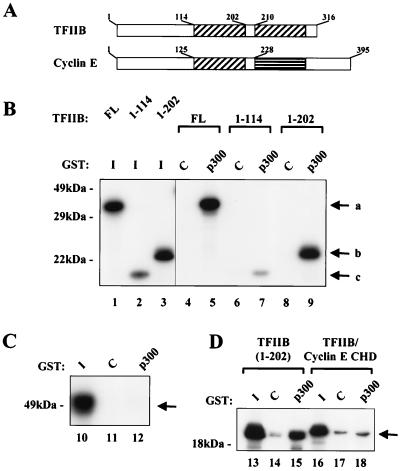
FIG. 3.
The cyclin homology domain of TFIIB but not cyclin E interacts with p300. (A) Both TFIIB and cyclin E contain cyclin homology domains. The two cyclin homology domains in TFIIB and the one in cyclin E are indicated by hatched boxes. The amino acid end points of the relevant domains in both proteins are indicated. (B) The cyclin homology domain and the amino terminus of TFIIB interact with p300. In vitro-translated fragments of TFIIB, including the entire protein (FL), a form truncated at amino acid 114 (1-114), and a form truncated at amino acid 202 (1-202), were incubated with control GST (C) or GST-p300 (p300). Complexes were analyzed by SDS–12% PAGE, and lanes 1 to 3 were used to show 10% of the in vitro-translated products. The GST-p300 fragment contained amino acids 1532 to 1900. Arrows labeled a, b, and c indicate the positions of TFIIB amino acids 1 to 316, 1 to 202, and 1 to 114, respectively. (C) Cyclin E alone does not bind to p300. In vitro-translated cyclin E was incubated with control GST (lane 11) or GST-p300 (lane 12). Complexes were examined by SDS–12% PAGE. Lane 10 contains 10% of the in vitro-translated cyclin E. (D) A chimeric protein containing the amino terminus of TFIIB and the cyclin homology domain of cyclin E does not bind to p300. Control GST (lanes 14 and 17) or GST-p300 (lanes 15 and 18) was incubated with the in vitro-translated TFIIB fragment (amino acids 1 to 202) or a similar fragment in which the TFIIB cyclin homology domain was replaced with the cyclin E cyclin homology domain (TFIIB/cyclin E CHD). Complexes were analyzed by SDS–12% PAGE, and 10% of the input in vitro-translated proteins are shown in lanes 13 and 16.