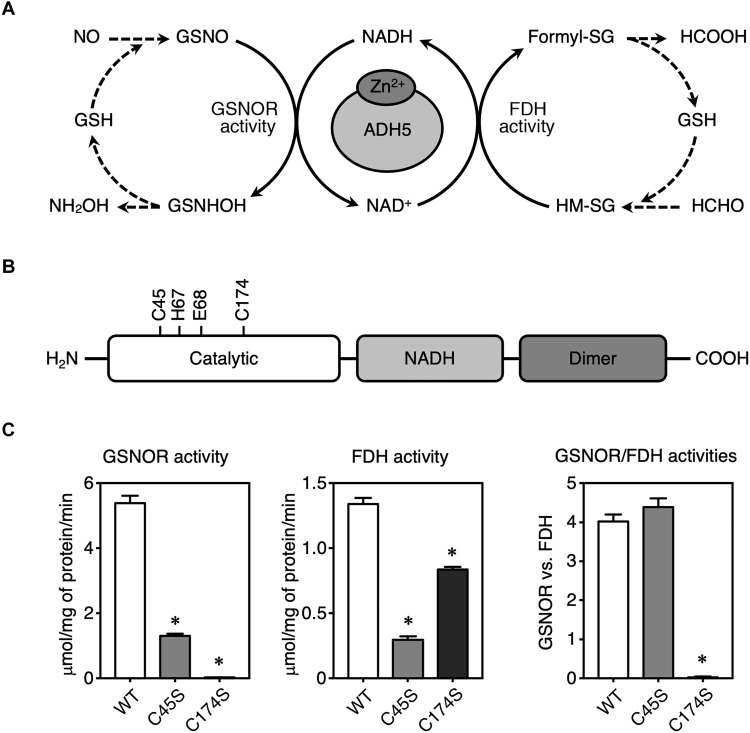
Fig. 1. Illustration of the GSNOR and FDH activities of recombinant human WT ADH5 protein and Cys mutants of ADH5.
(A) Schematic showing reactions catalyzed by ADH5 with dual enzymatic activities—GSNOR and FDH. NH2OH, hydroxylamine; formyl-SG, S-formylglutathione; HCOOH, formic acid; HCHO, formaldehyde. (B) Domain structure of human ADH5 (hADH5) protein. (C) Measurement of enzymatic activities of recombinant WT hADH5 and mutants of hADH5. GSNOR (left) and FDH (middle) activities of ADH5 (WT, C45S, and C174S) proteins. The ratio of GSNOR activities to FDH activities was calculated for each ADH5 protein (right). The results are presented as means ± SD of three independent enzyme assays. *P < 0.001 versus WT; one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test.