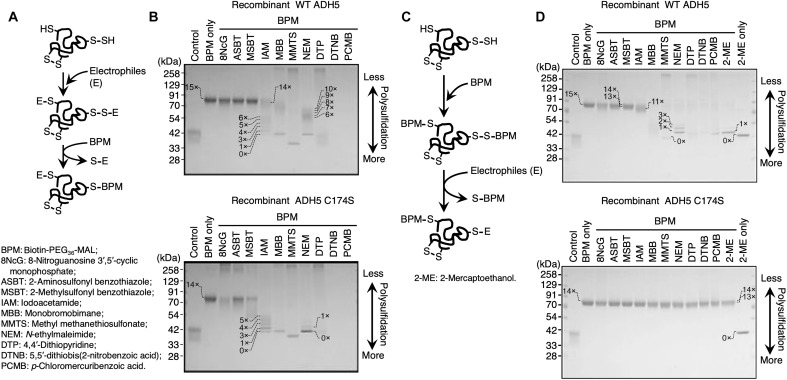
Fig. 2. Supersulfidation of recombinant hADH5 protein and its C174S mutant.
(A) Schematic showing the reaction mechanism in the original PMSA. In the initial step of the reaction, both thiol (─SH) and polysulfide [─(S)n-H] groups of the Cys residues in a protein are alkylated by the electrophiles (E). In the second reaction step, the PEGylation probe BPM selectively attacks alkylated polysulfides at proximal sulfur atoms. Polysulfide-rich proteins acquire PEGylation and demonstrate low gel mobility. (B) Detection of protein supersulfidation in WT ADH5 (top) and C174S mutant (bottom) proteins by using the original PMSA. Numbers on the gels and blots indicate expected numbers of PEG moieties in the protein, which were deduced from the mobility shift distance. Proteins were detected by means of CBB staining. 8NcG, 8-nitroguanosine 3′,5′-cyclic monophosphate; ASBT, 2-aminosulfonyl benzothiazole; MSBT, 2-methylsulfonyl benzothiazole; IAM, iodoacetamide; MBB, monobromobimane; MMTS, methyl methanethiosulfonate; NEM, N-ethylmaleimide; DTP, 4,4′-dithiopyridine; DTNB, 5,5′-dithiobis(2-nitrobenzoic acid); PCMB, p-chloromercuribenzoic acid. (C) Schematic drawing for the modified PMSA. In the initial step of the reaction, both thiol (─SH) and polysulfide [─(S)n-H] groups of the Cys residues in a protein are PEGylated by means of the PEGylation probe BPM. In the second reaction step, strong electrophilic compounds, but not weak electrophilic compounds, selectively attack PEGylated polysulfides at proximal sulfur atoms, which results in the removal of PEG moieties. Polysulfide-rich proteins lose PEGylation and exhibit high gel mobility by reacting with strong electrophiles. (D) Detection of protein supersulfidation in WT hADH5 (top) and C174S mutant (bottom) hADH5 proteins by using the modified PMSA.