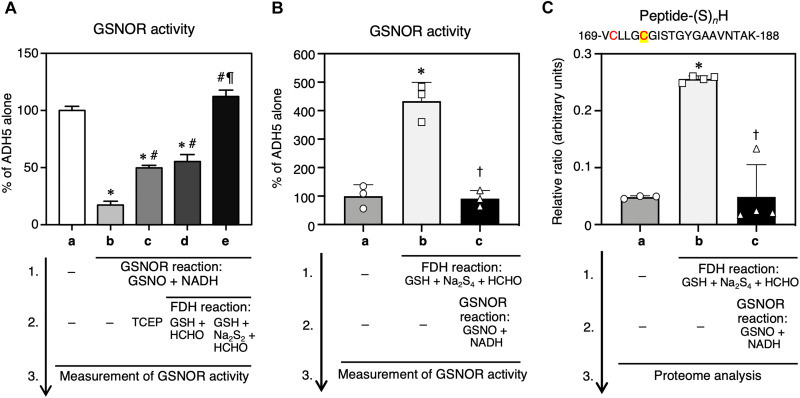
Fig. 5. Correlation of active site supersulfidation with GSNOR activity of ADH5.
Recombinant WT ADH5 was immobilized on Sepharose beads and then underwent sequential GSNOR and FDH reactions in the presence of the indicated substrates. (A) Effects of ADH5 enzyme reactions on the GSNOR activity of recombinant ADH5. The ADH5 protein was first treated with the GSNOR reaction mixtures (GSNO plus NADH) for 60 min at 37°C and then subjected to sequential FDH reactions, followed by measurement of GSNOR activity. The FDH reaction mixture included 1 mM NAD+ and HM-SG (100 μM GSH + 500 μM formaldehyde), HM-(S)n-SG (100 μM GSH + 500 μM formaldehyde + 50 μM Na2S2), or a reducing agent (1 mM TCEP). (B) Effects of ADH5 enzyme reactions on the GSNOR activity of recombinant ADH5. The ADH5 protein was first treated with the FDH reaction mixtures containing 100 μM NAD+ and a substrate cocktail of HM-(S)n-SG (10 μM GSH + 50 μM formaldehyde + 5 μM Na2S4) and then subjected to sequential GSNOR reactions, followed by measurement of GSNOR activity. (C) High-precision sulfur proteome analysis of supersulfidated Cys residues of ADH5. The recombinant ADH5 that were treated with sequential FDH and GSNOR reactions, after which supersulfides formed at the GSNOR catalytic center was identified via the sulfur proteome. A peptide fragment containing Cys residues C170 (red) and C174 (red and yellow) is shown in the panel headline. The ADH5 alone (a) served as a control for other reactions (b to e). Results are presented as means ± SD (n = 3 to 4). *P < 0.001 versus ADH5 alone (A-a, B-a, and C-a), #P < 0.001 versus GSNOR (A-b), ¶P < 0.001 versus GSNOR + FDH (GSH + HCHO) (A-d), †P < 0.001 versus FDH (B-b and C-b); one-way ANOVA with Tukey’s multiple comparison test.