Abstract
A body of evidence suggests that exposure to psychosocial stressors and stress sensitivity are involved in psychosis pathogenesis. However, little is known about the temporal course of these domains in those with psychosis-risk syndromes. Furthermore, to date, there have been no studies examining associations between psychosocial stressors and impaired stress tolerance, or how these factors might be implicated in symptom progression prior to psychosis onset. A total of 73 clinical high-risk (CHR) participants and 78 healthy controls (HCs) completed baseline measures of life event (LE) exposure and impaired stress tolerance. Additionally, 54 CHR and 57 HC participants returned to complete the same procedures at a 12-month follow-up assessment. Results indicated that when compared to HCs, CHR individuals exhibited increased LE exposure and impaired stress tolerance at baseline. Longitudinal analyses compared subgroups of CHR participants who exhibited positive symptoms worsening over the 1-year course (CHR-Prog), improved or steady (CHR-Remiss/Persist), and HCs. CHR-Prog individuals showed consistently elevated independent LEs exposure while CHR-Remiss/Persist reported a decline and HCs a steady low level across time. Furthermore, CHR-Prog exhibited increased stress intolerance, while the CHR-Remiss/Persist improved and HCs displayed consistently low levels over time. Analyses examining interrelationships between these domains showed a trend level interaction effect predicting follow-up symptoms. Taken together, results from the present study indicate an important role for exposure to stressors and increasing stress intolerance during psychosis pathogenesis. Additionally, findings indicating that decreases in stress exposure may lead to more favorable outcomes provide a promising target for novel targeted interventions.
Keywords: independent life events, stress sensitivity, schizophrenia, positive symptoms
Introduction
The roles of psychosocial stressors and stress sensitivity in the etiology of psychotic disorders have been well established in the literature.1–6 In this context, a number of prominent investigations have provided evidence to suggest that exposure to a greater number of recent life events (LEs) stressors, independent from illness and outside of a person’s control, is related to higher relapse rates for psychotic disorders.1,2 Dovetailing this work is an increasingly well-informed understanding of stress sensitivity, which reflects the subjective experience as well as vulnerability in the psychological and biological response systems.7–9 Yet, the field lacks a clear understanding of what role these 2 domains play in individuals with psychosis-risk syndromes, such as those at Clinical High Risk (CHR) for psychosis. While a handful of studies have gathered valuable evidence informing our understanding of exposure to LEs in CHR individuals,6,10–13 longitudinal perspectives have been scarce, as have studies incorporating both LE exposure and stress tolerance. Examining these domains over time, understanding patterns associated with changes in symptoms, and exploring inter-relationships between stress exposure and sensitivity can provide a more nuanced understanding of pathogenic factors for psychosis and inform targeted intervention efforts.
Evidence suggests that LEs are a good indicator of psychosocial stress in formal psychosis, and some studies tie elevated exposure to relapse.1–3,14 There is also some support for LEs uniquely predicting transition from high-risk state to psychosis.10 Some studies have found that the number of LEs correlates with positive symptoms11,12,15 and identified LE exposure as predictive of symptom worsening over time in CHR individuals.6 However, CHR literature presents with some inconsistencies regarding LE exposure rates and their impact on symptomatology. While the majority of existing studies found significant differences between CHR individuals and healthy controls (HCs),6,10,11 others have reported a comparable or lower number of LEs compared to HCs.16,17 Moreover, no studies have thoroughly examined the LE exposure over time. A longitudinal perspective and examination of LE exposure in the context of other components of stress may help explain noted inconsistencies and provide insights into mechanisms contributing to psychosis onset.
While LEs are an important index of the psychosocial environment of a given person, they do not provide direct information about the individual experience or stress response systems. As noted, stress sensitivity or intolerance to stressors is another component that has been highlighted as mechanistically important by the literature.9,13 This domain is highly relevant as it addresses a tricky factor for stress research. Specifically, the experience of stress is highly subjective, and exposure to a given stressor may affect one person differently than another.16,18,19 Therefore, directly querying about stress sensitivity may help avoid a number of confounds inherent in a system placing value rankings on what constitutes a stressor. Additionally, reports of intolerance to stress are likely to reflect vulnerability in biological and psychological stress and coping/resiliency systems.7–9 Consistent with neural diathesis-stress and stress-cascade models, any changes in this experience may reflect pathogenic mechanisms driving illness.5,20
However, while the domain is conceptually interesting, the empirical studies to advise our understanding in this important area have been scarce, particularly in the psychosis-risk populations. The existing studies indicate that impaired stress tolerance may be associated with increased positive symptoms in CHR individuals.13,21 Furthermore, when compared to HCs, CHR youth present with higher levels of impairment, suggesting that stress sensitivity is affected in the early stages of psychosis development and indicative of vulnerability prior to onset.8,13,22 Studies have also shown that at this stage, subjective ratings of daily stressors are elevated.6,23 In the context of CHR populations, stress intolerance may prove particularly effective as a measure that is less vulnerable to individual differences in responses to stressors (in a highly heterogeneous population) and sensitive to emerging illness. Furthermore, understanding it over time provides a good opportunity to understand changes in biological and resiliency systems which may be implicated in driving the illness.
Investigating exposure to psychosocial stressors in relation to impaired stress tolerance can provide valuable insights into how these distinct domains together may be involved in illness progression. There is some evidence that individuals with schizophrenia with high stress reactivity are more impacted by LE exposure24; however, it is still unclear how these 2 factors together impact illness progression. Prominent theoretical models linking these domains together suggest that vulnerable psychological and biological systems becoming increasingly taxed by psychosocial stressors during the psychosis-risk period and further damaged by accompanying persistent stress become increasingly less capable of marshaling homeostatic mechanisms.20 Indeed, a Cascade model predicts the subsequent changes to stress sensitivity as a key pathogenic factor.5,25 A recent study found that CHR individuals endorsed greater distress surrounding LE exposure even when exposure was lower than in HC individuals,16 implicating stress sensitivity as a possible mechanism amplifying the effects of exposure. Although emerging cross-sectional evidence suggests that stress sensitivity and exposure to LEs together may be predictive of symptoms,12 investigations of the impact of enduring exposure and changes in stress tolerance over time have been lacking.
To address these questions, the present investigation first sought to replicate previous findings of elevated LE exposure6,10,11 and stress intolerance8,13,22 in CHR by assessing baseline differences between HC and CHR. We predicted that CHR individuals will report elevated exposure to independent LEs and present with significantly more impaired stress tolerance when compared to HCs. Then, a longitudinal perspective was adopted to determine the pattern of LE exposure and stress tolerance across time and relative to illness status in CHR subgroups experiencing worsening of positive symptoms over time (progression of psychosis-risk syndrome [CHR-Prog]) and improvement or steady course of symptoms (remission or persistence of psychosis-risk syndrome [CHR-Remiss/Persist]), and HCs. As noted, previous research suggests exposure to LEs is associated with worsening of illness,1–3,10,14 and a recent study indicated that impaired stress tolerance is associated with symptoms over time.13 Therefore, we predicted that the CHR-Prog group will experience an increase in LE exposure and more impaired stress tolerance while CHR-Remiss/Persist will improve and HCs report consistent low levels in both domains over a 1-year period. Finally, we examined the relationship between stress intolerance and independent LE exposure in predicting positive symptoms in the CHR-Prog group. We predicted that individuals who endorse the most impaired stress tolerance will be more affected by elevated LE exposure.
Methods
Participants
Participants were recruited at the Adolescent Development and Preventive Treatment Program, and the sample included 73 CHR (female 30, male 43, mean age 18.62, SD = 1.77) and 78 HC (female 44, male 34, mean age 18.17, SD = 2.62) participants. CHR participants met criteria for the psychosis-risk syndrome by one or more of the following: (1) presence of attenuated psychosis symptoms, (2) presence of schizotypal personality disorder with a global functioning decline and age younger than 19, and (3) a family history of psychosis with global functioning decline. Exclusion criteria for all participants included age younger than 12 or older than 24, psychotic disorder diagnosis, history of head injuries and neurological disorders, and a lifetime diagnosis of substance use. Additional exclusionary criteria for HC participants included meeting CHR criteria and family history of psychosis. A subset of participants completed the study procedures at the 12-month follow-up (54 CHR and 57 HC participants). Out of these 54 CHR participants, 30 were characterized by symptom remission or persistence between time points (CHR-Remiss/Persist; defined as a decrease or no change in total positive symptoms) and 24 by symptom progression (CHR-Prog; defined as at least a 1-point increase in total positive symptoms; figure 1). In the Structured Clinical Interview for Psychosis Risk Syndromes (SIPS),26,27 the progression qualifier is defined as at least a scale point increase on any positive symptom compared to 12 months ago. More specifically, a change from 3 to 4 on the SIPS-positive scale indicates an increase in related distress and possible interference in functioning; a change from 4 to 5 indicates a lack of self-induced skepticism and significant interference with functioning; a change from 5 to 6 indicates frankly psychotic symptoms. Persistence and remission are defined by no change in symptoms and at least a scale point decrease, respectively.26
Fig. 1.
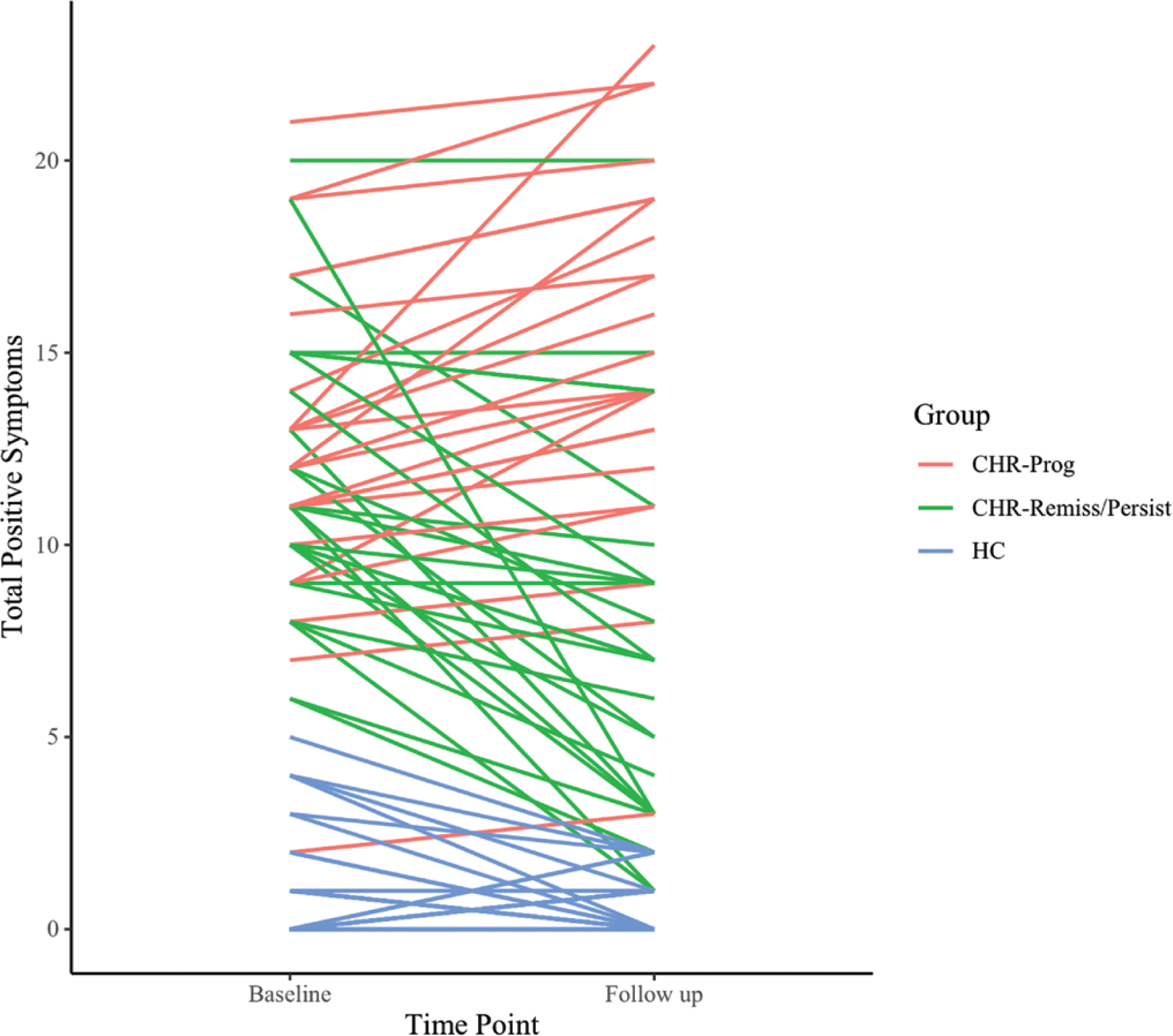
Positive symptoms progression from baseline to follow-up. CHR-Prog, clinical high-risk symptom progression group; CHR-Remiss/Persist, clinical high-risk symptom remission/persistence group; HC, healthy controls.
Clinical Assessments
The SIPS26,27 was employed to diagnose attenuated psychosis in CHR participants and rule out symptoms in HCs, and it was administered by trained assessors. The Structured Clinical Interview for DSM-IV (SCID)28 was administered to participants in both groups to rule out psychosis and assess for other psychiatric disorders. All psychodiagnostic interviews were completed by trained assessors who achieved reliability of at least k = 0.80 regarding SIPS and SCID symptoms, as well as 90% agreement on all diagnostic classifications across assessments.
Impaired Tolerance to Stress
The general SIPS scale was used to assess impaired tolerance to stress rated on a 7-point scale from “absent” to “extreme.” The scale measures increasing challenges and the inability to cope with daily activities and stressful situations.
LEs Assessment
The presence of LEs was assessed using a modified self-report scale adapted from the Psychiatric Epidemiology Research Interview (PERI) LEs Scale.29 The measure includes 59 events in 9 categories and has been previously used in research with adolescents.6,11,24 Based on prior research,2,14 LEs were categorized in terms of how much influence the participant had over their occurrence. The event was deemed independent if not related to psychosis-risk illness and beyond participants’ control or influence (see supplementary table 1). Events were deemed dependent if they were related to illness or in the scope of participants’ control (eg, getting married).30 Two independent raters categorized LEs on a 4-point scale: 0 = totally independent, 1 = possibly dependent, 2 = probably dependent, and 3 = definitely dependent,30 reaching inter-rater reliability κ = 0.86. Average ratings were used to determine LE status. LEs with an average rating of 1 were deemed too ambiguous and were excluded from all analyses to more accurately delineate between the event types. Given the findings in psychosis literature suggesting specificity of effects to independent LEs,2,31 the focus of the current study is on independent LEs and how they relate to other stress measures and symptomatology.
Statistical Approach
Z tests (Skewness/SEskewness and Kurtosis/SEkurtosis) were used to evaluate the normality of the data. Due to the evidence of non-normality indicated by z values greater than 1.99 based on the current sample size,32 analyses were performed with log-transformed LE and stress intolerance variables. Independent samples t-tests, one-way ANOVAs, and chi-square tests were used to test differences in continuous and categorical demographic and symptom variables, respectively. To examine the pattern of reported independent LEs across time points between groups, a univariate ANOVA with Bonferroni-corrected post-hoc analysis was performed for each time point. Group differences in impaired stress tolerance were tested using the same analyses. Finally, it was of interest to increase understanding of candidate underlying mechanisms by exploring how LE and stress tolerance interact in the context of illness trajectory. Given the results indicating between-group differences for these 2 distinct stress domains and associations of their trajectories with illness progression in the CHR-Prog group only, a regression analysis was conducted to determine whether the interaction between independent LEs exposure and impaired stress tolerance predicted positive symptoms in the CHR-Prog group at follow-up (controlling for baseline symptom values).
Results
Sample Characteristics
The CHR and HC groups did not significantly differ in demographic information including age (t(149) = 1.23, P = .22), gender (χ2(1, N = 151) = 3.54, P = .06), and parental education (t(149) = 0.22, P = .82). As expected, the CHR group reported significantly more positive symptoms at baseline (t(149) = 21.31, P < .0001) and follow-up (t(109) = 11.65, P < .0001; table 1). There were no significant differences in age (F(2,110) = 0.75, P = .47), gender (χ2(1, N = 111) = 4.94, P = .08), or parental education (F(2,110) = 0.20, P = .82) between the 3 groups. Additionally, CHR-Prog, CHR-Remiss/Persist, and HC groups also did not differ in age (F(2,110) = 0.75, P = .47), gender (χ2(1, N = 151) = 4.94, P = .08), and parental education (F(2,110) = 0.12, P = .82; table 2). At baseline, a small proportion of CHR participants reported use of antipsychotics (12.3% at baseline and 15.1% at follow-up). The direction and magnitude of results for the analyses did not change when employing antipsychotics as a covariate, so the following sections are presented without it.
Table 1.
Demographic and symptom characteristics by group at baseline
| CHR | HC | p | |
|---|---|---|---|
|
| |||
| Gender | |||
| Males | 43 | 34 | |
| Females | 30 | 44 | |
|
|
|||
| Total | 73 | 78 | NS |
| Age | |||
| Mean years (SD) | 18.62 (1.77) | 18.17(2.62) | NS |
| Parental education | |||
| Mean years (SD) | 15.78 (2.32) | 15.69 (2.82) | NS |
| Positive Symptoms | |||
| Mean (SD) | 11.89 (4.48) | .60 (1.30) | ** |
Note: NS=not significant
p<.0001
CHR=Clinical High-Risk; HC=Healthy Controls
Table 2.
Demographic, symptom, life event, and stress intolerance characteristics by group across time points
| Baseline | Follow-up | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| CHR-Prog | CHR-Remiss/Persist | HC | p | CHR-Prog | CHR-Remiss/Persist | HC | p | |
|
| ||||||||
| Gender | ||||||||
| Males | 16 | 17 | 25 | |||||
| Females | 14 | 7 | 32 | |||||
|
|
||||||||
| Total | 30 | 24 | 57 | NS | ——— | ——— | ——— | — |
| Age | ||||||||
| Mean years (SD) | 18.30 (1.88) | 18.67 (1.83) | 17.98 (2.70) | NS | ——— | ——— | ——— | — |
| Parental education | ||||||||
| Mean years (SD) | 15.80 (2.40) | 16.19 (2.11) | 15.86 (2.54) | NS | ——— | ——— | ——— | — |
| Positive Symptoms | ||||||||
| Mean (SD) | 10.53 (4.86) | 12.54 (4.19) | .58 (1.23) | ** | 6.53 (4.90) | 15.08 (4.89) | .30 (.63) | ** |
| Independent Life Events | ||||||||
| Mean (SD) | 1.57 (1.34) | 1.33 (1.55) | .82 (.93) | * | .55 (.69) | 1.14 (1.21) | .52 (.69) | * |
| Impaired Tolerance to Stress | ||||||||
| Mean (SD) | 1.93 (1.54) | 1.88 (1.45) | .09 (.34) | ** | 1.37 (1.47) | 2.29 (1.43) | .12 (.331) | ** |
Note: NS = not significant
p <.05
p < .0001 for omnibus effect
CHR-Prog=Clinical high-risk symptom progression group; CHR-Remiss/Persist=Clinical high-risk symptom remission or persistence group; HC=Healthy controls.
Baseline Differences Between CHR and HC Participants in Independent LE Exposure and Stress Tolerance
Consistent with the predictions, the univariate ANOVAs demonstrated that compared to the HC group, CHR participants experienced significantly more independent LEs (F(1,150) = 12.31, P = .001, η2(partial) = 0.08) with a medium effect size. Additionally, tolerance to stress was significantly more impaired in the CHR group (F(1,150) = 137.69, P < .0001, η2(partial) = 0.48) with a large effect size, whereas the CHR group reported impairment levels higher than expected in response to daily stressors, and HCs reported little to no difficulties with coping with stressful situations.
Changes in Independent LE Exposure Across Time
The univariate ANOVAs examining the differences in independent LEs at each time point between CHR subgroups and the HCs indicated significant omnibus effects with medium effect sizes at baseline (F(2,110) = 3.44, P = .04, η2(partial) = 0.06) and at follow-up (F(2,104) = 3.41, P = .04, η2(partial) = 0.06). Furthermore, post-hoc analyses revealed that the 2 CHR groups reported an almost identical amount of independent LEs at baseline (95% CI [−0.23, 0.47, P = 1]) and that only the HC and the CHR-Remiss/Persist groups were significantly different (95% CI [−0.59, −0.01, P = .04]). Interestingly, at follow-up, the number of independent LEs decreased for the CHR-Remiss/Persist group, which was not the case for CHR-Prog. Specifically, the CHR-Prog group reported significantly more events than HCs (95% CI [0.01, 0.56], P = .04) and, a trend toward more than the CHR-Remiss/Persist group (95% CI [−0.04, 0.57], P = .11). Meanwhile, the CHR-Remiss/Persist group reported a comparable number of events to the HCs (95% CI [−0.27, 0.23], P = 1; figure 2).
Fig. 2.
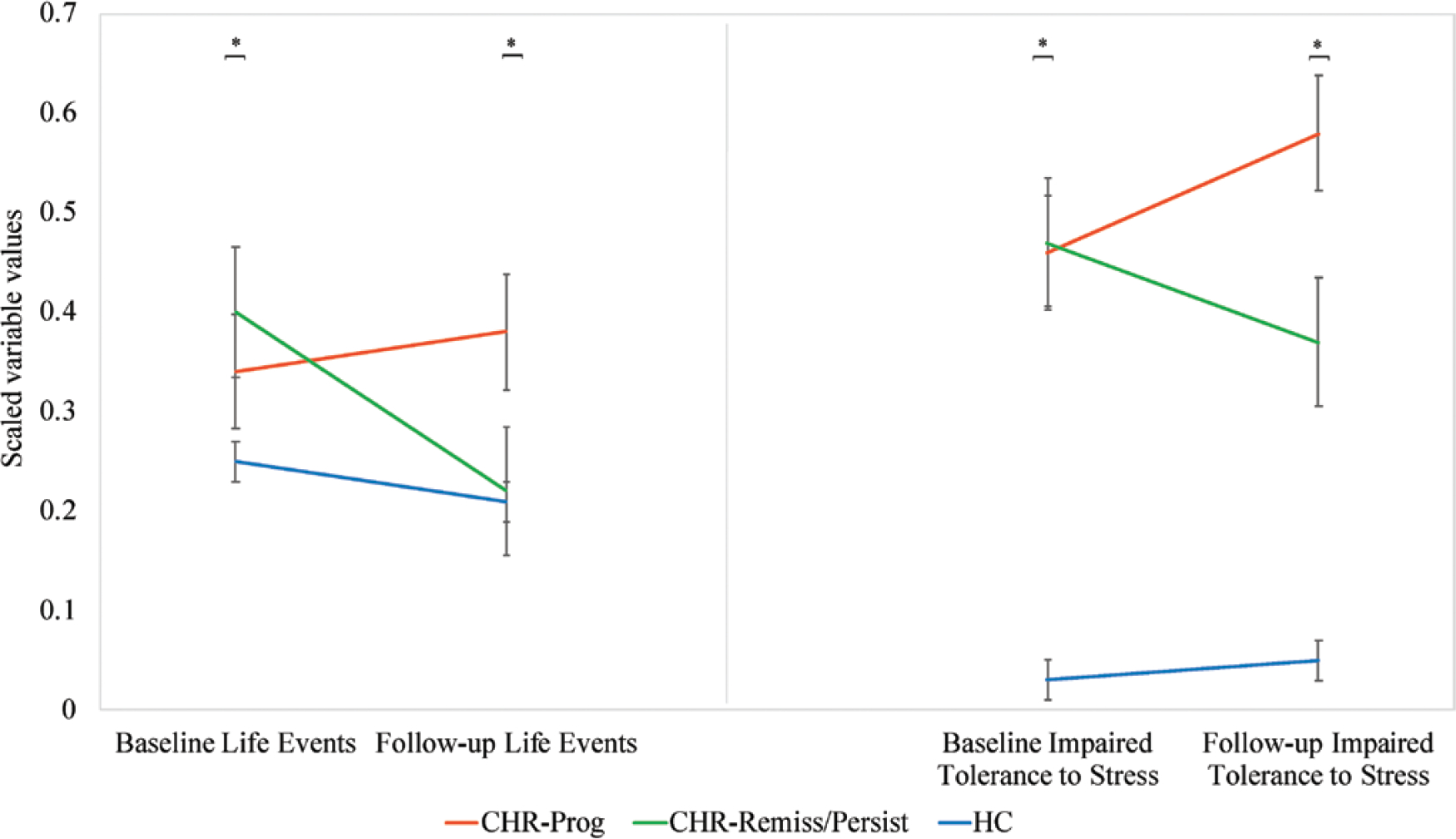
Independent life events and stress intolerance over time. *P < .05 for omnibus effect for between-group differences. CHR-Prog, clinical high-risk symptom progression group; CHR-Remiss/Persist, clinical high-risk symptom remission/persistence group; HC, healthy controls.
Differences in Stress Intolerance Across Time
Omnibus effects for stress intolerance were significant at baseline (F(2,110) = 50.72, P < .0001, η2(partial) = 0.48) and at follow-up (F(2,110) = 41.49, P < .0001, η2(partial) = 0.43). Furthermore, at baseline, while the HC group reported significantly higher stress tolerance than both CHR groups, CHR-Remiss/Persist and CHR-Prog presented with the same level of impairment (95% CI [−0.29, 0.31, P = 1]). However, at follow-up, the CHR-Remiss/Persist group experienced a significant improvement in stress tolerance while the CHR-Prog group presented with more impaired tolerance to stress. As expected, the HC group remained significantly different from both CHR groups. Post-hoc analyses revealed a significant difference between CHR-Remiss/Persist and CHR-Prog groups (95% CI [−0.69, 0.07, P = .01]; figure 2). Critically, the CHR-Prog group that reported sustained exposure to independent LEs also reported a worsening in stress tolerance between time points.
The Relationship Between Independent LEs and Stress Intolerance in Predicting Positive Symptoms at Follow-up in the CHR-Prog Group
A trend level interaction effect was observed for LE exposure and stress intolerance on symptom severity (β = −.4, t(17) = −1.88, P = .08). The pattern suggests that for those participants who endorsed greater than average stress intolerance, the degree of exposure to independent LEs was not as predictive of positive symptoms (figure 3). However, for those with less impaired tolerance to stress (sample average or below-average levels), a greater number of endorsed LEs were more impactful in predicting higher levels of positive symptoms.
Fig. 3.
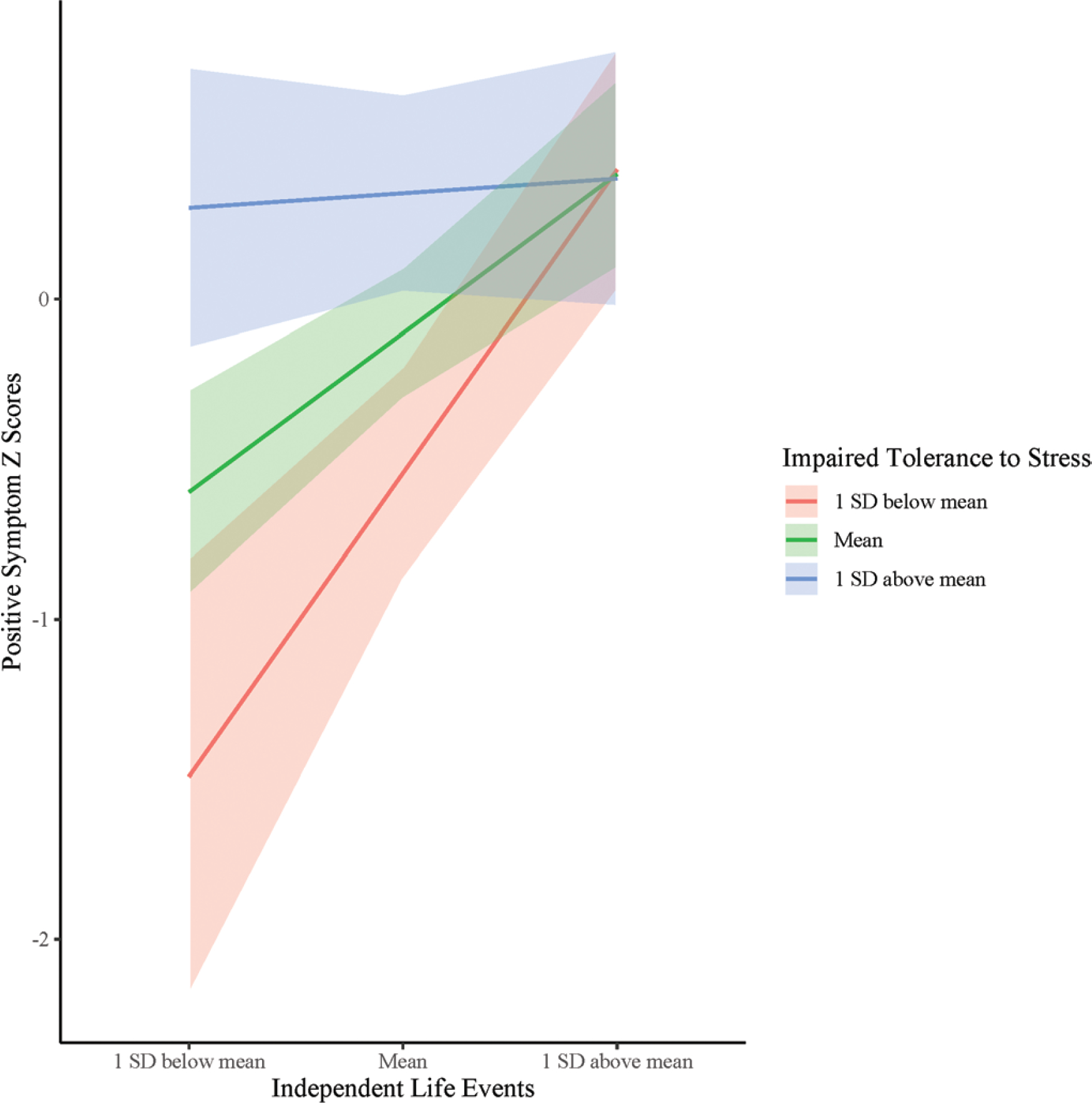
Independent life events × stress intolerance interaction at follow-up in the CHR-Prog group.
Discussion
The present study expanded our understanding of psychosocial stressors and stress sensitivity in youth with a psychosis-risk syndrome. First, the findings replicated a small but growing number of studies in observing that the broad CHR group experienced more LEs6,10,11 and greater impaired tolerance to stress8,13,22 when compared to HCs. Second, longitudinal analyses indicated that independent LEs remain constant over time in CHR individuals with increasingly more severe positive symptoms over time, but that these stressful experiences remit in those CHR individuals who show improvement or maintain a steady course of positive symptoms. This is consistent in some ways with findings that independent LEs are related to the course of illness in formal psychosis2,11,31 but the first direct evidence to support an important role for clinical progression in CHR youth. It is also noteworthy that we observed a pattern for increasing stress intolerance in the CHR-Prog group, while the CHR-Remiss/Persist group and HCs showed decreasing or steady patterns, respectively. Taken together, these results speak to an important pathogenic role for psychosocial stressors and increasingly impaired stress sensitivity. Finally, analyses aimed at determining interrelationships between the 2 domains provide a novel and potentially important perspective of a dynamic interplay between external stressors and internal experience/stress sensitivity. Specifically, trending results suggested that LE exposure and stress intolerance interact to predict positive symptoms.
Considering CHR individuals are particularly affected by exposure to stressors, establishing whether LE rates are elevated in this population is crucial for determining whether they play a role in illness pathogenesis. As mentioned, the literature is mixed on the rates of exposure to LEs in both formal psychosis2,3,14,33 and CHR6,11,16,17 literature. The inconsistency may be due to differences across studies in focus on exposure to total LEs vs different LE types.6 While many CHR studies looked at cumulative LEs, the present approach focused on independent LEs. The importance of this approach is that, considering the independent LEs occur outside of an individual’s control, we can more confidently infer that exposure may indeed be a risk factor rather than a by-product of the illness. Consistent with studies reporting elevated psychosocial stress exposure,6,11 the results indicated that compared to the HCs, the CHR group endorsed more baseline LEs. Elevated rates in the CHR group suggest that external stressors are etiologically informative as they are evident prior to illness onset. Additionally, the degree of subjective stress may be playing a role in how susceptible this population is to the effects of exposure to psychosocial stressors.4,6,34 Both schizophrenia and CHR literature notes aberrant stress responsiveness.9,13,34,35 In line with these findings, and consistent with predictions, the baseline analyses revealed a significantly impaired stress tolerance in the CHR group when compared to HCs providing additional support for the early vulnerability of the stress systems.
Longitudinal analyses of independent LEs indicated distinct exposure trajectories for CHR subgroups. Specifically, the results indicated that independent LE exposure over a 1-year period may relate to illness progression. This was inconsistent with findings from an earlier study by DeVylder et al13 which reported that exposure to LEs across time was not associated with positive symptoms in CHR. While this study investigated total LEs, the focus of the present study was on independent LEs that could be driving the differences in findings. Additionally, DeVylder et al observed CHR participants over the average of 4 time points suggesting the timeline may play a role in the extent to which exposure to LEs contributes to illness progression. The results revealed that although the CHR groups reported nearly identical baseline exposure rates, the amount of independent LEs significantly decreased for the CHR-Remiss/Persist group, matching the rates endorsed by HCs. Contrary to the prediction, the CHR-Prog group endorsed consistent high-level exposure rather than an elevation suggesting that chronic exposure may indeed be a risk factor for positive symptom worsening. Although the difference between the CHR groups was at a trend level, the persistent LE trajectory in the group experiencing the worsening of symptoms (figure 2) provides compelling evidence for the involvement of independent LEs in the course of illness. Specifically, chronic exposure, even when not elevated across time, may be related to symptom worsening while a decrease is associated with more favorable outcomes possibly suggesting that changes in levels of impairment of the stress system may play a role in mechanisms by which LE exposure affects symptoms.
In regards to the longitudinal course of stress intolerance, the analyses provided further support that stress sensitivity is an important pathogenic mechanism. A previous study found a temporal relationship between stress sensitivity and symptoms,13 and the current investigation expanded the research by examining the progression of stress intolerance in distinct CHR subtypes. Specifically, while impairment levels were comparable at baseline, as hypothesized, the groups were significantly different at follow-up, wherein the CHR-Remiss/Persist group reported improvement while the CHR-Prog group experienced more impairment. As expected, the HC group endorsed consistent low-level impairment over time. These findings indicate that the internal experience of stress is impacted early in the course of illness and may be reflective of the increasing vulnerability of psychological and biological stress systems. Interestingly, the CHR group that experienced a worsening in stress tolerance also endorsed enduring exposure to independent LEs indicating these distinct stress domains may be a part of the shared mechanism contributing to illness progression.
The analysis examining the relationship between these 2 domains lends some support for the independent LEs and stress tolerance interaction in predicting positive symptoms. As predicted, at trend levels, the degree of stress tolerance impairment interacted with independent LE exposure to predict symptom worsening. However, contrary to the prediction that those at highest levels of impairment would be the most affected by LE exposure, the pattern indicated there may be a ceiling effect as to the extent to which these 2 domains interrelate with regard to predicting symptom progression. Specifically, while at above-average impairment in stress tolerance the amount of independent LEs was not as related to stress impairment, at average and below-average levels a greater number of independent LEs were particularly predictive of more severe positive symptoms (figure 3). Considering the vast heterogeneity in clinical presentations in psychoses as suggested by Dickinson et al,36 future research employing cluster analyses based on the degree of impairment in CHR could help elucidate which patient subgroups may be more impacted by environmental stressors and stress systems vulnerability prior to illness onset.
These findings are consistent with the diathesis-stress and stress-cascade models of psychosis which posit that existing vulnerability interacts with environmental stress to ultimately drive psychosis onset.5,20,25,37,38 Animal models of schizophrenia show abnormal stress responsivity in rats.39–41 One study found that juvenile rats treated with an agent mimicking abnormalities associated with schizophrenia exhibit exaggerated behavioral responses and blunted hypothalamic-pituitary-adrenal axis (HPA) response to acute stressors and the inability to adapt to chronic exposure.39 Other promising mechanisms by which stress tolerance could be affected by LE exposure include atrophy to hippocampal subregions42–45 occurring via HPA axis dysregulation.6,13,20 Therefore, chronic exposure is particularly important to consider as consistent psychosocial insults may be conferring risk and increasing sensitivity to stress. Taken together, this confluence of persistent psychosocial stressors and increasing stress sensitivity may impact brain structure/function and subsequently cognition and coping through epigenetic modulatory effects,46,47 affect normative dopamine transmission,48–50 and ultimately, drive the onset of psychosis. Current findings provide additional support for these models. The results indicate that consistent LE exposure is related to worsening of symptoms and that together with stress tolerance (indexing subjective experience and psychobiological vulnerability) is a key factor driving the progression of the illness. Additionally, examining LE exposure in relation to stress tolerance in early stages of the illness is critical because adolescence and young adulthood are periods characterized by increasingly demanding environments51,52 and neuromaturational processes.53–55 In CHR individuals, persistent psychosocial stress may be impacting an already vulnerable system rendering it less adept to self-regulate and adjust to environmental changes, thereby further conferring risk and contributing to a psychotic outcome.
Finally, the current investigation presents both strengths and limitations. The current approach to defining the course of illness has been used in previous studies to examine nuanced changes in symptomatology.56 However, whether or not a 1-point change represents a definitive shift remains an empirical question. Future studies with larger samples should investigate clinical outcome as a dichotomous (transition/remission) variable to draw more conclusive inferences. Furthermore, although we used a well-established LE measure that has been used with adolescent populations in previous studies, the PERI has not been validated for use with adolescents. In addition, future studies could benefit from a more stringent timeline of exposure (such as utilizing a timeline followback method) and corroborative information to address issues related to self-report measures. Furthermore, a more comprehensive measure of stress sensitivity could yield stronger results. Although the retrospective design allowed us to assess LEs longitudinally and capture exposure over a 1-year period leading up to each assessment, future investigations would benefit from utilizing prospective designs. Additionally, the trend level result (which is comparable to/large in comparison to what has been previously reported, but small when considering multisite studies in this area) may be due to the low sample size in the CHR-Prog group. Therefore, incorporating larger samples and utilizing longitudinal mixed models would help in establishing stronger findings. Taken together, findings are poised to inform future investigations surrounding etiology, indicating opportunities for the development of targeted interventions.
Supplementary Material
Funding
This work has been supported by the National Institute of Mental Health (grant numbers R01MH112545-01, R01MH116039-01A1, R21/R33 Award, MH103231, R21 MH115231 to [V.A.M.]).
Footnotes
Conflicts of Interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin Open online.
References
- 1.Malla AK, Cortese L, Shaw TS, Ginsberg B. Life events and relapse in schizophrenia. A one year prospective study. Soc Psychiatry Psychiatr Epidemiol. 1990;25(4):221–224. [DOI] [PubMed] [Google Scholar]
- 2.Ventura J, Nuechterlein KH, Lukoff D, Hardesty JP. A prospective study of stressful life events and schizophrenic relapse. J Abnorm Psychol. 1989;98(4):407–411. [DOI] [PubMed] [Google Scholar]
- 3.Mansueto G, Faravelli C. Recent life events and psychosis: the role of childhood adversities. Psychiatry Res. 2017;256:111–117. [DOI] [PubMed] [Google Scholar]
- 4.Holtzman CW, Shapiro DI, Trotman HD, Walker EF. Stress and the prodromal phase of psychosis. Curr Pharm Des. 2012;18(4):527–533. [DOI] [PubMed] [Google Scholar]
- 5.Corcoran C, Walker E, Huot R, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29(4):671–692. [DOI] [PubMed] [Google Scholar]
- 6.Trotman HD, Holtzman CW, Walker EF, et al. Stress exposure and sensitivity in the clinical high-risk syndrome: initial findings from the North American Prodrome Longitudinal Study (NAPLS). Schizophr Res. 2014;160(1–3):104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McEwen BS. Biomarkers for assessing population and individual health and disease related to stress and adaptation. Metabolism. 2015;64(3 suppl 1):S2–S10. [DOI] [PubMed] [Google Scholar]
- 8.Pruessner M, Iyer SN, Faridi K, Joober R, Malla AK. Stress and protective factors in individuals at ultra-high risk for psychosis, first episode psychosis and healthy controls. Schizophr Res. 2011;129(1):29–35. [DOI] [PubMed] [Google Scholar]
- 9.Myin-Germeys I, van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27(4):409–424. [DOI] [PubMed] [Google Scholar]
- 10.Walker E Independent and joint predictive power of life events, daily stress, and cortisol in relation to conversion to psychosis. Psychoneuroendocrinology. 2016;71:9. [Google Scholar]
- 11.Tessner KD, Mittal V, Walker EF. Longitudinal study of stressful life events and daily stressors among adolescents at high risk for psychotic disorders. Schizophr Bull. 2011;37(2):432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson LE, Anglin DM, Klugman JT, et al. Stress sensitivity mediates the relationship between traumatic life events and attenuated positive psychotic symptoms differentially by gender in a college population sample. J Psychiatr Res. 2014;53:111–118. [DOI] [PubMed] [Google Scholar]
- 13.DeVylder JE, Ben-David S, Schobel SA, Kimhy D, Malaspina D, Corcoran CM. Temporal association of stress sensitivity and symptoms in individuals at clinical high risk for psychosis. Psychol Med. 2013;43(2):259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown GW, Birley JL. Crises and life changes and the onset of schizophrenia. J Health Soc Behav. 1968;9(3):203–214. [PubMed] [Google Scholar]
- 15.Johns LC, Cannon M, Singleton N, et al. Prevalence and correlates of self-reported psychotic symptoms in the British population. Br J Psychiatry. 2004;185:298–305. [DOI] [PubMed] [Google Scholar]
- 16.Phillips LJ, Edwards J, McMurray N, Francey S. Comparison of experiences of stress and coping between young people at risk of psychosis and a non-clinical cohort. Behav Cogn Psychother. 2012;40(1):69–88. [DOI] [PubMed] [Google Scholar]
- 17.Kraan T, Velthorst E, Smit F, de Haan L, van der Gaag M. Trauma and recent life events in individuals at ultra high risk for psychosis: review and meta-analysis. Schizophr Res. 2015;161(2–3):143–149. [DOI] [PubMed] [Google Scholar]
- 18.Weinberger DA, Schwartz GE, Davidson RJ. Low-anxious, high-anxious, and repressive coping styles: psychometric patterns and behavioral and physiological responses to stress. J Abnorm Psychol. 1979;88(4):369–380. [DOI] [PubMed] [Google Scholar]
- 19.Sommerfeldt SL, Schaefer SM, Brauer M, Ryff CD, Davidson RJ. Individual differences in the association between subjective stress and heart rate are related to psychological and physical well-being. Psychol Sci. 2019;30(7):1016–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. [DOI] [PubMed] [Google Scholar]
- 21.Corcoran CM, Smith C, McLaughlin D, Auther A, Malaspina D, Cornblatt B. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr Res. 2012;135(1–3):170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Steen Y, Gimpel-Drees J, Lataster T, et al. Clinical high risk for psychosis: the association between momentary stress, affective and psychotic symptoms. Acta Psychiatr Scand. 2017;136(1):63–73. [DOI] [PubMed] [Google Scholar]
- 23.Pruessner M, Béchard-Evans L, Boekestyn L, Iyer SN, Pruessner JC, Malla AK. Attenuated cortisol response to acute psychosocial stress in individuals at ultra-high risk for psychosis. Schizophr Res. 2013;146(1–3):79–86. [DOI] [PubMed] [Google Scholar]
- 24.Docherty NM, St-Hilaire A, Aakre JM, Seghers JP. Life events and high-trait reactivity together predict psychotic symptom increases in schizophrenia. Schizophr Bull. 2009;35(3):638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corcoran C, Malaspina D, Goetz R, Gil R, Gorman J, McEwen B. Elements of the stress cascade and the neurobiology of schizophrenia. Schizophr Res. 2003;60(1):13.12505134 [Google Scholar]
- 26.McGlashan T, Walsh B, Woods S. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-up. New York: Oxford University Press; 2010. [Google Scholar]
- 27.Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. [DOI] [PubMed] [Google Scholar]
- 28.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). New York: Biometrics Research, New York State Psychiatric Institute; 2012. [Google Scholar]
- 29.Dohrenwend BS, Krasnoff L, Askenasy AR, Dohrenwend BP. Exemplification of a method for scaling life events: the PERI Life Events Scale. J Health Soc Behav. 1978;19(2):205–229. [PubMed] [Google Scholar]
- 30.Safford SM, Alloy LB, Abramson LY, Crossfield AG. Negative cognitive style as a predictor of negative life events in depression-prone individuals: a test of the stress generation hypothesis. J Affect Disord. 2007;99(1–3):147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bebbington P, Wilkins S, Sham P, et al. Life events before psychotic episodes: do clinical and social variables affect the relationship? Soc Psychiatry Psychiatr Epidemiol. 1996;31(3–4):122–128. [DOI] [PubMed] [Google Scholar]
- 32.Kim HY. Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restor Dent Endod. 2013;38(1):52–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horan WP, Ventura J, Nuechterlein KH, Subotnik KL, Hwang SS, Mintz J. Stressful life events in recent-onset schizophrenia: reduced frequencies and altered subjective appraisals. Schizophr Res. 2005;75(2–3):363–374. [DOI] [PubMed] [Google Scholar]
- 34.Norman RM, Malla AK. Stressful life events and schizophrenia. I: a review of the research. Br J Psychiatry. 1993;162:161–166. [DOI] [PubMed] [Google Scholar]
- 35.Lee H, Schepp K. The relationship between symptoms and stress in adolescents with schizophrenia. Issues Ment Health Nurs. 2009;30(12):736–744. [DOI] [PubMed] [Google Scholar]
- 36.Dickinson D, Pratt DN, Giangrande EJ, et al. Attacking heterogeneity in schizophrenia by deriving clinical subgroups from widely available symptom data. Schizophr Bull. 2018;44(1):101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104(4):667–685. [DOI] [PubMed] [Google Scholar]
- 38.Zubin J, Spring B. Vulnerability—a new view of schizophrenia. J Abnorm Psychol. 1977;86(2):103–126. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerman EC, Bellaire M, Ewing SG, Grace AA. Abnormal stress responsivity in a rodent developmental disruption model of schizophrenia. Neuropsychopharmacology. 2013;38(11):2131–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giovanoli S, Engler H, Engler A, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339(6123):1095–1099. [DOI] [PubMed] [Google Scholar]
- 41.Gomes FV, Grace AA. Prefrontal cortex dysfunction increases susceptibility to schizophrenia-like changes induced by adolescent stress exposure. Schizophr Bull. 2017;43(3):592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Erp TG, Saleh PA, Rosso IM, et al. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry. 2002;159(9):1514–1520. [DOI] [PubMed] [Google Scholar]
- 43.Mittal VA, Walker EF. Minor physical anomalies and vulnerability in prodromal youth. Schizophr Res. 2011;129(2–3):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vargas T, Dean DJ, Osborne KJ, et al. Hippocampal subregions across the psychosis spectrum. Schizophr Bull. 2018;44(5):1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collip D, Habets P, Marcelis M, et al. Hippocampal volume as marker of daily life stress sensitivity in psychosis. Psychol Med. 2013;43(7):1377–1387. [DOI] [PubMed] [Google Scholar]
- 46.Millan MJ. An epigenetic framework for neurodevelopmental disorders: from pathogenesis to potential therapy. Neuropharmacology. 2013;68:2–82. [DOI] [PubMed] [Google Scholar]
- 47.Diwadkar VA, Bustamante A, Rai H, Uddin M. Epigenetics, stress, and their potential impact on brain network function: a focus on the schizophrenia diatheses. Front Psychiatry. 2014;5:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pani L, Porcella A, Gessa GL. The role of stress in the pathophysiology of the dopaminergic system. Mol Psychiatry. 2000;5(1):14–21. [DOI] [PubMed] [Google Scholar]
- 49.Brunelin J, d’Amato T, van Os J, Cochet A, Suaud-Chagny MF, Saoud M. Effects of acute metabolic stress on the dopaminergic and pituitary-adrenal axis activity in patients with schizophrenia, their unaffected siblings and controls. Schizophr Res. 2008;100(1–3):206–211. [DOI] [PubMed] [Google Scholar]
- 50.Howes OD, McCutcheon R, Owen MJ, Murray RM. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salusky I, Larson RW, Griffith A, et al. How adolescents develop responsibility: what can be learned from youth programs. J Res Adolesc. 2014;24(3):417–430. [Google Scholar]
- 52.Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Health. 2018;2(3):223–228. [DOI] [PubMed] [Google Scholar]
- 53.Fuhrmann D, Knoll LJ, Blakemore SJ. Adolescence as a sensitive period of brain development. Trends Cogn Sci. 2015;19(10):558–566. [DOI] [PubMed] [Google Scholar]
- 54.Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16(8):1412–1425. [DOI] [PubMed] [Google Scholar]
- 55.Keshavan MS, Giedd J, Lau JY, Lewis DA, Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1(7):549–558. [DOI] [PubMed] [Google Scholar]
- 56.Dean DJ, Orr JM, Bernard JA, et al. Hippocampal shape abnormalities predict symptom progression in neuroleptic-free youth at ultrahigh risk for psychosis. Schizophr Bull. 2016;42(1):161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


