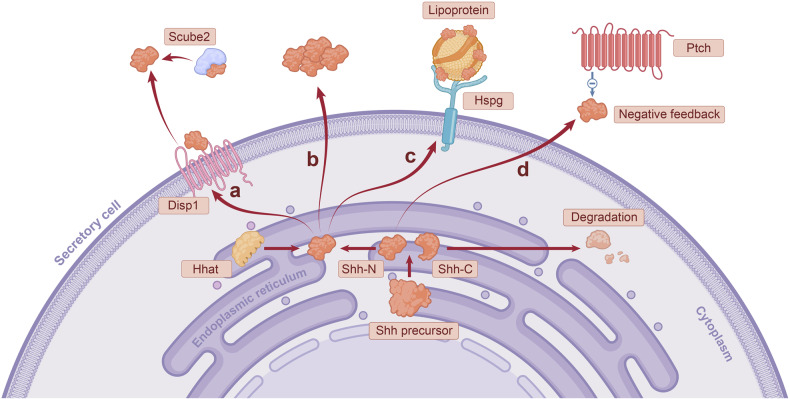
Fig. 1.
Activation and spread of hedgehog ligands in secretory cells. The three vertebrate hedgehog ligands Shh, Dhh and Ihh have similar secretion processes, differing slightly in the tissue distribution of the three. The figure shows the secretion of Shh as an example of the secretion process of hedgehog ligands. Activation of Shh occurs in the endoplasmic reticulum (ER). The Shh precursor is first processed into two parts, Shh-N and Shh-C. The carboxyl terminus of Shh-N is bound to cholesterol by a protein hydrolytic cleavage process, while the amino terminus of Shh-N is bound to palmitic acid mediated by Hhat. In contrast, Shh-C is degraded after being transported out of the ER. There are several mechanisms for the diffusion of Shh-N out of secretory cells after activation: a Shh-N is mainly transported out of secretory cells by the synergistic action of Disp1 and Scube2. b The polymerization of monomer-activated Shh-N into multimolecules facilitates the diffusion of ligands. c Hspg is localized to the secretory cell membrane, which recruits lipoprotein. Shh-N is loaded on the lipoprotein as a "passenger" for long-distance transportation. d Ptch1 on the surface of the receiving cells has a negative feedback regulation on the release of Shh-N