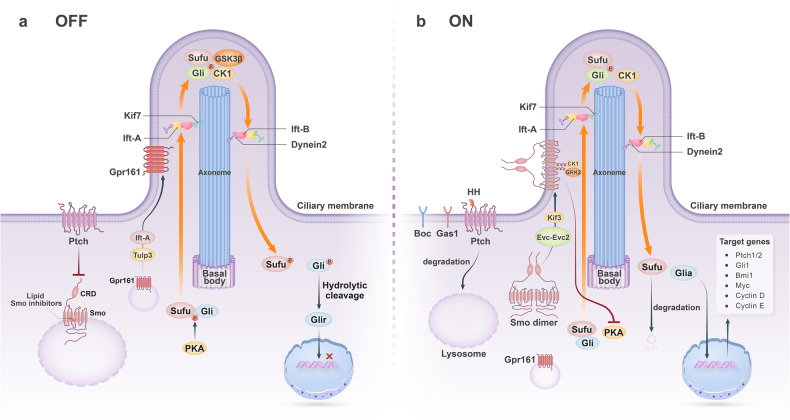
Fig. 3.
Primary cilia play a key role in HH signaling pathway. Primary cilia are composed of ciliary membrane, axoneme and basal body. The proper functioning of HH signaling relies on the Ift mechanism. Ift-B complex protein and Ift-A complex protein together constitute Ift trains that carry motors with HH signaling components sliding on the axoneme. Kinesin mediates transport from the base to the tip of the cilium, while dynein2 mediates transport from the tip to the base of the cilium. a Receptor cells are closed to signal transduction. In the absence of hedgehog ligand, Ptch1 inhibits Smo. Lipid Smo inhibitors bind to the TMD region of Smo, inhibit Smo activity, and constrain Smo to the cytoplasm. Gpr161 localizes to the cilia in the presence of Ift-A and Tulp3. Gpr161 induces PKA activity, and PKA phosphorylates the Sufu/Gli complex. Kinesin Kif7 then transports the complex to the cilia tip. Sufu is further phosphorylated by GSK3β; Gli is further phosphorylated by CK1 and GSK3β. The complex then dissociates and Gli is processed by protein hydrolytic cleavage to Glir, which subsequently enters the nucleus to repress target gene transcription. b Receptor cells are open to signal transduction. In the presence of Hedgehog ligand, the inhibition of Smo by Ptch is released and Ptch is transported to the lysosome for degradation. The co-receptors Boc and Gas1 can interact with Ptch1 and Ptch2 to form a receptor complex respectively, thereby promoting or inhibiting signal transduction. The concentration of intracellular lipid Smo inhibitor ligands decreases and Smo forms an activated dimeric form that is transported by Kinesin Kif3 and Evc-Evc2 proteins to the cilia membrane near the basolateral. The Smo carboxyl terminus is then fully activated by phosphorylation of CK1 and GRK2. After Smo activation, Gpr161 returns to the cytoplasm. Meanwhile, activated Smo inhibits PKA activity and is not sufficient to phosphorylate the Sufu/Gli complex. The unphosphorylated complex is transported to the cilia tip by Kinesin Kif7. The unphosphorylated Sufu is degraded by ubiquitination; Gli is phosphorylated by CK1 to form Glia. Glia enters the nucleus to promote the transcription of related target genes, such as Ptch1/2, which acts as a negative regulator of signal transduction, Gli1, which amplifies signals, Bmi1, which encodes a transcriptional repressor, and Myc, Cyclin D, and Cyclin E, which encode cell cycle regulators