Fig. 2.
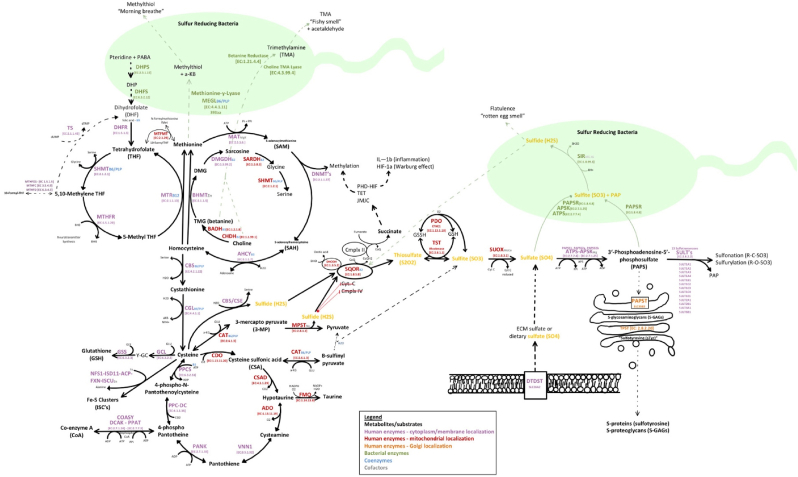
–In humans, sulfur pathways require sufficient intake of methionine along with adequate B-vitamins (B2, B3, B5, B6, B7, B9, B12), and trace elements zinc, nickel, molybdenum, cobalt, potassium, magnesium, iron, calcium, and sodium (Table 1) [4,6,7,19,68,71,77,174]. Methionine is essential as it can regenerate critical organic sulfur compounds, including the universal methyl donor s-adenosyl-l-methionine (SAM), homocysteine, cysteine, glutathione (GSH), iron-clusters (Fe–S), coenzyme A (CoA), hypotaurine, and taurine [4]. As a by-product of transsulfuration pathways, inorganic sulfur species (ISS) are produced. Specifically, the Cystathionine β-synthase (CBS), cystathionine γ-lyase (CGL), and 3-mercaptopyruvate sulfurtransferase (MST) enzymes produce endogenous H2S, mitochondrial SQOR, ETHE1/PDO, and TST enzymes produce SO32− and S2O32−, and mitochondrial SUOX produces endogenous SO42. Additionally, extracellular sulfate can also be transported into cells by SLC26A1 or SLC26A2 (DTDST) sulfate/chloride antiporters [63]. The oxidation of inorganic sulfur species (H2S, SO32− and S2O32−) then occurs step-wise in the mitochondria where thiosulfate and GSSH are oxidized by either iron-dependent persulfide dioxygenase (PDO/ETHE1) or by thiosulfate sulfur transferase (TST/rhodanese) to produce sulfite (SO32−) and regenerate reduced glutathione (GSH) [40,42,43,45,47,49,175]. The final and most oxidized inorganic sulfur species produced from sulfite (S = +4), is inorganic sulfate (SO42−) (S = +6), by molybdenum-dependent sulfite oxidase (SOUX), which resides in the intermembrane space of the mitochondria [54]. Sulfate is readily used as a substrate for sulfonation/sulfurylation reactions after activation to 3′-phosphoadenosine-5′-phosphosulfate (PAPS) by PAPS synthase [13,14].