Table 2.
Copper-catalyzed asymmetric 1,4-hydrosilylation of 1,3-enynesa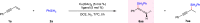
| entry | ligand (5 mol %) | T/ oC | t/h | 6aa | 7aa | |
|---|---|---|---|---|---|---|
| yield % | ee % | yield % | ||||
| 1 | L1* | 40 | 1 | 44 | 1 | trace |
| 2 | L2* | 40 | 1 | 10 | −12 | 3 |
| 3 | L3* | 40 | 1 | 24 | −19 | trace |
| 4 | L4* | 40 | 1 | 10 | −32 | 1 |
| 5 | L5* | 40 | 1 | 54 | 43 | trace |
| 6 | L5* | 0 | 1 | 42 | 68 | trace |
| 7 | L5* | −10 | 1 | 23 | 73 | trace |
| 8 | L5* | −20 | 2 | 38 | 79 | trace |
| 9 | L5* | −30 | 11 | 60 | 85 | trace |
| 10 | L5* | −50 | 11 | 75b | 96 | trace |
| 11 | L5* | −50 | 11 | 78 (72)b,c,d | 96 | trace |
aReaction conditions: Cu(OAc)2 (5 mol %), ligand (5 mol %), 1a (0.2 mmol) and 2a were stirred in DCE (1 mL) under nitrogen atmosphere, the yields were determined by crude 1H NMR using 1,1,2,2-tetrachlorethane as an internal standard.
b1a and 2a were added under −35 °C.
cDCE = 2 mL, PhSiH3 = 2.0 equiv.
dIsolated yield.
