Figure 2. Mechanisms regulating gasdermin D-mediated cell death.
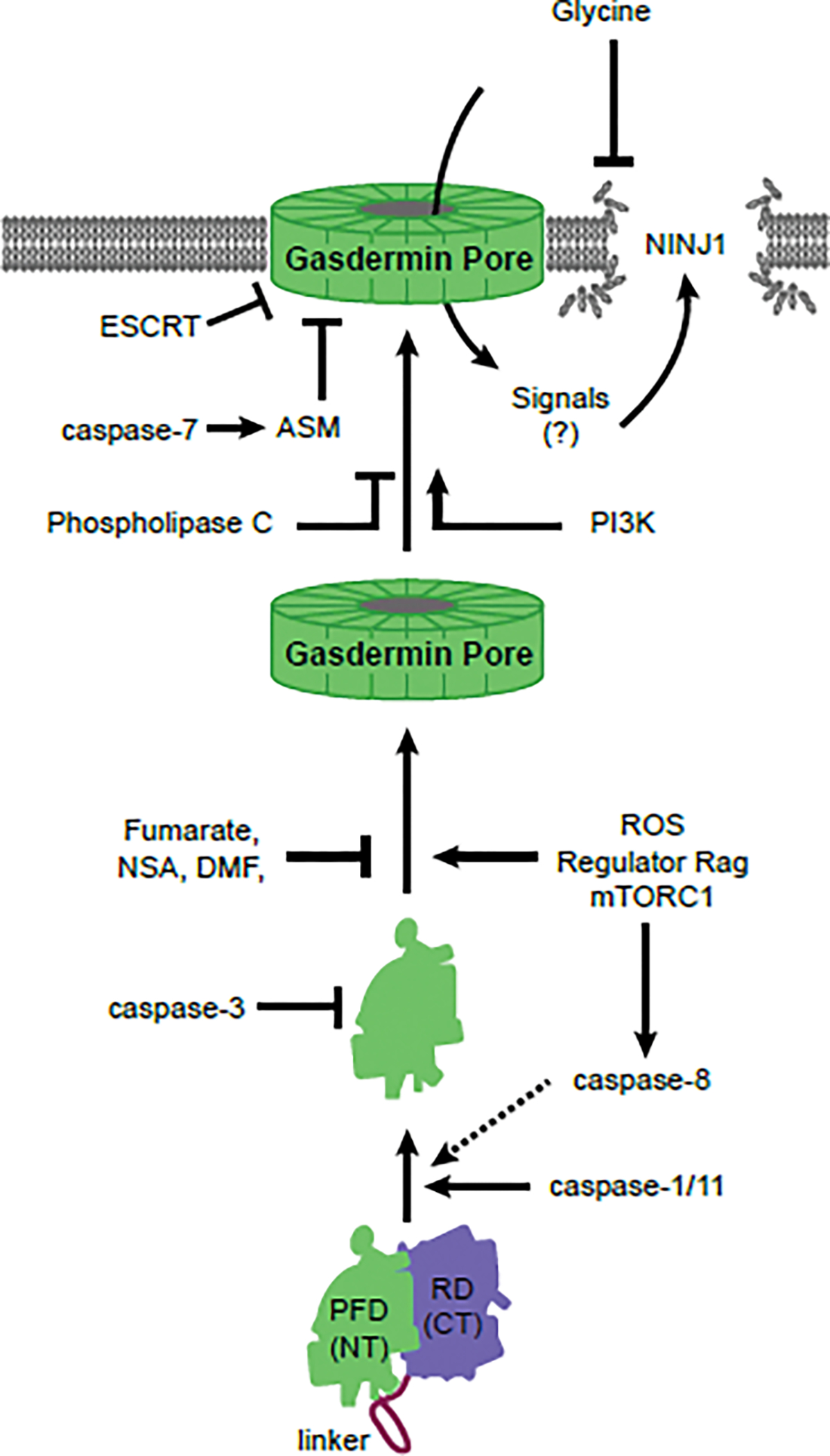
The process of gasdermin D-mediated pyroptosis is strictly regulated. Before activation, the C-terminal (CT) regulatory domain (RD) of gasdermin D seal and inhibit the N-terminal (NT) pore forming domain (PFD). Caspase-1/11 (or caspase-8 slowly) can cleave gasdermin D, releasing the active PFD that oligomerizes into large pore complexes. In parallel, gasdermin D can be cleaved by caspase-3 into an inactive form. During oligomerization, active gasdermin D PFDs require a critical cysteine to remain unmodified to permit oligomerization via ROS generating activation of Regulator Rag complex-mTORC1. Several drugs (fumarate, NSA, DMF) or intrinsic metabolites such as succinate derivative inhibit the availability of this cysteine. After pore formation, ESCRT and/or ASM-driven membrane repair pathways can remove the pores form the plasma membrane. Phosphoinositide-3 kinase (PI3K) and phospholipase C can modulate membrane phosphoinositide composition to alter the pore-opening/closing dynamics. After gasdermin D pores open, ninjurin-1 (NINJ1) causes plamsa membrane rupture. Extracellular glycine applied to cells can inhibit plasma membrane rupture in a manner that is similar to the phenotype of Ninj1-deficient cells.
