Abstract
Introduction
COPD is underdiagnosed, and measurement of spirometry alongside low-dose computed tomography (LDCT) screening for lung cancer is one strategy to increase earlier diagnosis of this disease.
Methods
Ever-smokers at high risk of lung cancer were invited to the Yorkshire Lung Screening Trial for a lung health check (LHC) comprising LDCT screening, pre-bronchodilator spirometry and a smoking cessation service. In this cross-sectional study we present data on participant demographics, respiratory symptoms, lung function, emphysema on imaging and both self-reported and primary care diagnoses of COPD. Multivariable logistic regression analysis identified factors associated with possible underdiagnosis and misdiagnosis of COPD in this population, with airflow obstruction defined as forced expiratory volume in 1 s/forced vital capacity ratio <0.70.
Results
Out of 3920 LHC attendees undergoing spirometry, 17% had undiagnosed airflow obstruction with respiratory symptoms, representing potentially undiagnosed COPD. Compared to those with a primary care COPD code, this population had milder symptoms, better lung function and were more likely to be current smokers (p≤0.001 for all comparisons). Out of 836 attendees with a primary care COPD code who underwent spirometry, 19% did not have airflow obstruction, potentially representing misdiagnosed COPD, although symptom burden was high.
Discussion
Spirometry offered alongside LDCT screening can potentially identify cases of undiagnosed and misdiagnosed COPD. Future research should assess the downstream impact of these findings to determine whether any meaningful changes to treatment and outcomes occur, and to assess the impact on co-delivering spirometry on other parameters of LDCT screening performance such as participation and adherence. Additionally, work is needed to better understand the aetiology of respiratory symptoms in those with misdiagnosed COPD, to ensure that this highly symptomatic group receive evidence-based interventions.
Tweetable abstract
Measurement of spirometry alongside lung cancer screening identifies possible undiagnosed COPD in 17% of participants; conversely, 19% of people with a known diagnosis of this condition may have been misdiagnosed https://bit.ly/3Jhh0OR
Introduction
COPD is a worldwide health problem [1] and is widely recognised to be underdiagnosed [2–4]. Despite this, there remains debate as to the effectiveness of active screening or case-finding. Neither the United Kingdom (UK) National Screening Committee nor the United States Preventive Service Taskforce (USPSTF) recommend screening asymptomatic or mildly symptomatic adults for COPD [5, 6]. In comparison, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines advocate active case-finding in those with symptoms and/or risk factors, alongside proactive identification and management of other coexisting comorbidities [7]. Furthermore, the UK National Institute for Health and Care Excellence guidelines suggest spirometry for patients with computed tomography (CT) or chest radiography evidence of emphysema or chronic airways disease [8].
Lung cancer screening was approved in the United States in 2013 following publication of the National Lung Screening Trial which showed reduced lung cancer mortality associated with low-dose CT (LDCT) screening compared with chest radiography [9]. The NELSON study [10] confirmed the benefit of LDCT screening in reducing lung cancer mortality, and many nations are now considering implementing comprehensive screening programmes. In 2022, the UK National Screening Committee recommended targeted screening in those aged 55–74 years identified as being at high risk of lung cancer [11] These targeted screening programmes arguably provide an opportunity for active case-finding for COPD. Previous UK pilot studies have invited individuals to a lung health check (LHC) comprising LDCT scans and other interventions such as smoking cessation and spirometry [12, 13]. Here we present outcomes from spirometry in a community-based LHC delivered as part of the Yorkshire Lung Screening Trial (YLST) and identify determinants of possibly undiagnosed or misdiagnosed COPD.
Methods
Yorkshire Lung Screening Trial study design
The full protocol of the YLST has been published previously [14]. People aged 55–80 years resident in Leeds (UK) were invited to a telephone-based risk-assessment. Those at high risk of lung cancer according to any of three eligibility criteria (USPSTF; Liverpool Lung Project V.2; the model derived from the Prostate Lung Colorectal and Ovarian screening study, known as PLCOM2012) were invited for a LHC including a LDCT scan which took place in mobile units at convenient community locations. YLST was approved by a research ethics committee (reference 18/NW/0012) and is registered with the ISRCTN (reference ISRCTN42704678).
Spirometry
YLST participants were offered spirometry as part of the LHC, performed by trained staff using a handheld device, Vitalograph Pneumotrac. Measurements of pre-bronchodilator forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were performed in line with the Association for Respiratory Technology & Physiology guidelines [15]. Due to the rapid throughput of participants through the mobile screening units, it was considered impracticable to offer post-bronchodilator spirometry in this setting due to the longer time required to collect these measurements and thus the likely disruption to participant flow as a result. Reference values with percentage predicted based on the Global Lung Function Initiative were included, as well as observed and predicted FEV1/FVC ratio. Airflow obstruction was defined as FEV1/FVC ratio <0.7; lower limit of normal values were not recorded. Baseline recruitment to YLST took place between November 2018 and February 2021. Spirometry was stopped in March 2020 due to the coronavirus disease 2019 pandemic and was not re-started.
Data collection
General practitioner (GP) codes for a previous diagnosis of COPD were extracted from primary care electronic healthcare records (information on the robustness of this diagnosis was not available, i.e. lung function testing or symptoms at time of diagnosis). All other parameters were self-reported. Participants were asked whether they had a known history of COPD, emphysema or asthma. COPD Assessment Test (CAT) score, modified Medical Research Council (mMRC) dyspnoea score, World Health Organization (WHO) performance status and the presence of COPD-defining symptoms (exertional breathlessness, chronic cough, regular sputum production, wheeze, frequent winter bronchitis) [16] were recorded. The number of hospital admissions for chest problems, number of antibiotic courses for chest infections in the past 12 months and any prescribed inhaled medications were also documented.
LDCT scans and radiological reporting
LDCT scans for YLST are reported by a consortium of consultant thoracic radiologists. Scans are reported using Veolity (MeVis), a bespoke software package for lung cancer screening including automated volumetry and computer-aided detection. The presence and characteristics of emphysema (extent, type, distribution) based on visual assessment by the reporting radiologist were recorded for baseline scans using a standardised template within Veolity.
Communication of results and referral
A standardised letter communicating the results of the LDCT scan and spirometry was sent to the participant and primary care. Initially, all individuals with airflow obstruction, a self-reported respiratory symptom, and no GP COPD code were referred to the community healthcare trust for assessment. Due to service capacity concerns, this policy was revised in January 2019 with referrals restricted to the criteria described earlier and an FEV1 <80% predicted.
Statistical analysis
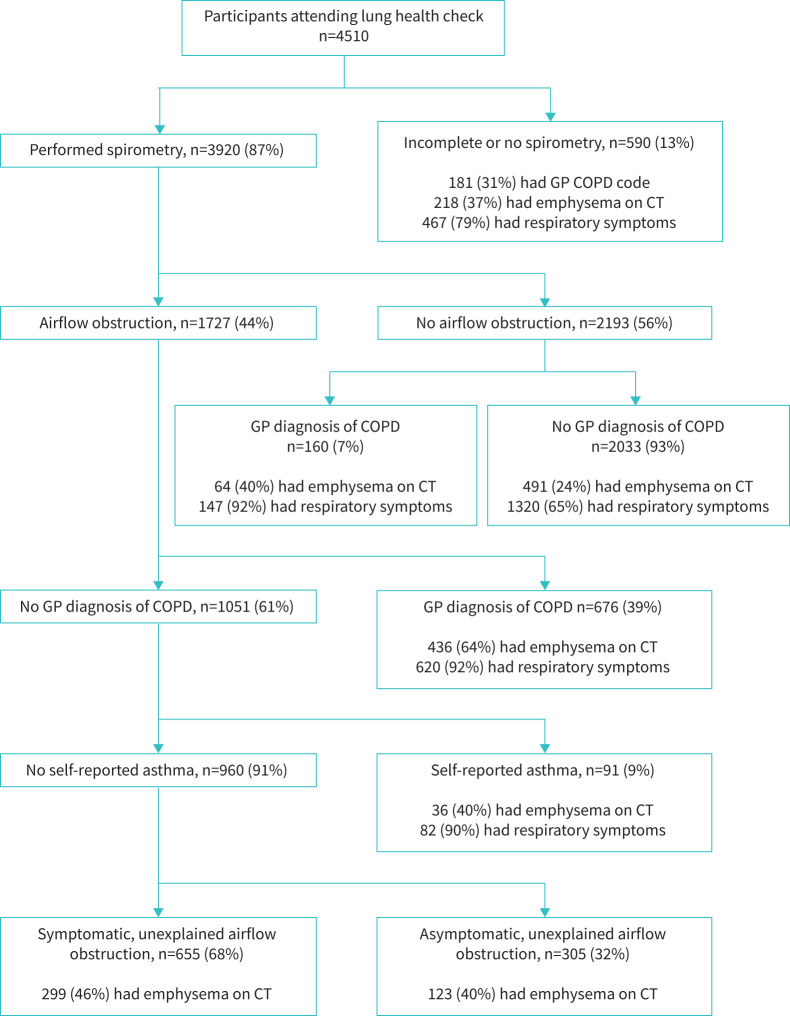
Participants were categorised into one of five groups for analysis (figure 1): airflow obstruction and a GP COPD code; airflow obstruction, no GP COPD code and at least one COPD-defining symptom (possible undiagnosed COPD); airflow obstruction, no GP COPD code and asymptomatic; no airflow obstruction but a GP COPD code (possible misdiagnosed COPD); and neither airflow obstruction nor a GP COPD code. As asthma is an alternative explanation for airflow obstruction, participants self-reporting a diagnosis of asthma were excluded from the unexplained airflow obstruction groups.
FIGURE 1.
Flow chart showing patient categorisation according to spirometry result, general practitioner (GP) diagnostic codes for COPD, self-reported asthma and the presence of respiratory symptoms. COPD-defining symptoms were exertional breathlessness, chronic cough, regular sputum production, wheeze and frequent winter bronchitis as per the National Institute for Health and Care Excellence guideline [8]. CT: computed tomography.
Descriptive statistics for the five groups according to demographic, clinical and spirometry/CT findings, are outlined in supplementary tables E1, E2 and E3 (missing data listed where relevant). Between-group comparisons described in results sections were performed using a t-test or Mann–Whitney U-test, as appropriate. Multivariable analysis with logistic regression was performed to identify variables that discriminated between relevant groups. For this regression analysis, information on inhaled medication, spirometry and CT findings were not included as regression parameters, as they either defined the groups in question or were a downstream consequence of diagnosis. Variables were initially included with p<0.2, then in backwards regression eliminated if p>0.01 (it was decided a priori to keep age and sex in the model without being subject to elimination criteria to ensure these parameters were accounted for). Statistical analysis was performed using Stata 17.
Results
Between November 2018 and March 2020, 4510 participants attended for a LHC, of whom 1017 had a GP-coded diagnosis of COPD. There was generally good concordance between self-reported and GP-recorded diagnoses of COPD; 93% of those with a GP code self-reported COPD, while 89% of those who self-reported COPD or emphysema had a GP COPD code.
A flow chart with categorisation according to spirometry and other clinical information is shown in figure 1. No spirometry values were recorded for 590 participants, either because measurement was declined or contraindicated or a reliable measurement was not obtained. Compared to those with spirometry values recorded, those without a measurement were older, from more deprived populations, had a greater smoking history, worse symptoms and worse performance status (p<0.001 for all comparisons, data not shown).
As outlined in figure 1, of the 3920 participants with spirometry values recorded, 1727 (44%) had airflow obstruction (FEV1/FVC ratio <0.7). Of those with airflow obstruction, 676 (39%) had a GP diagnosis of COPD, of whom 56 reported no current symptoms. Of the 1051 individuals with airflow obstruction and no pre-existing COPD diagnosis, 91 self-reported asthma and were excluded from subsequent group analysis. The remaining 960 participants with unexplained airflow obstruction were divided into two groups depending on the presence (n=655) or absence (n=305) of COPD-defining symptoms. Thus, of all participants undergoing spirometry, 16.7% (655 out of 3920) had symptomatic airflow obstruction without a GP COPD code, and therefore had possible undiagnosed COPD. Of the 2193 participants who did not have airflow obstruction, 160 participants had a GP COPD code, potentially representing misdiagnosis of COPD. These 160 participants constituted 19% of the 836 participants with a GP diagnostic COPD code for whom spirometry was recorded. Supplementary tables E1, E2 and E3 present demographic, clinical and spirometry/CT information for the five groups described earlier.
We performed statistical comparisons between two groups of particular interest; those with possible undiagnosed COPD (n=655) and those with known confirmed COPD (n=676; airflow obstruction and GP code). Undiagnosed individuals were more likely to be male (59.2% versus 49.4%, p<0.001), and to currently smoke (48.7% versus 38.3%, p<0.001), but were less symptomatic as evidenced by lower (better) mMRC dyspnoea score, better WHO performance status and lower COPD CAT score (p<0.001 for all comparisons). Those with possible undiagnosed COPD had better spirometry (mean±sd) than participants with a known confirmed diagnosis (FEV1 2.02±0.61 L versus 1.61±0.59 L, p<0.001; FEV1 80±19% pred versus 69±19% pred, p<0.001; FEV1/FVC 62.7±6.1% pred versus 56.7±9.1% pred, p<0.001). Fewer participants with possible undiagnosed COPD had LDCT evidence of emphysema (mild/moderate/severe/very severe) compared to those with a known confirmed diagnosis (45.6% versus 64.5%, p=0.001), and emphysema, where present, tended to be milder in those without a diagnosis.
Multivariable logistic regression was undertaken to identify factors associated with the absence of a GP COPD code in those with airflow obstruction and COPD-defining symptoms (table 1). The factors that increased the likelihood of an absent diagnostic code were male sex and current smoking status. Factors that reduced the likelihood of an absent code were a higher COPD CAT score, a higher mMRC dyspnoea score and the presence of sputum or frequent winter bronchitis as recorded symptoms.
TABLE 1.
Multivariable logistic regression for factors predicting the absence of a general practice COPD diagnostic code among all those with airflow obstruction and COPD-defining symptoms (655 out of 1275)
| OR (95% CI) | p-value | |
| Age | 0.99 (0.97–1.01) | 0.16 |
| Sex (male) | 1.34 (1.04–1.72) | 0.024 |
| Smoking status (current smoker) | 1.59 (1.21–2.08) | 0.001 |
| Higher (worse) COPD CAT score | 0.95 (0.93–0.98) | <0.001 |
| Higher (worse) mMRC dyspnoea score | 0.67 (0.57–0.78) | <0.001 |
| COPD symptom: sputum | 0.65 (0.50–0.86) | 0.002 |
| COPD symptom: bronchitis | 0.50 (0.32–0.77) | 0.002 |
CAT: COPD Assessment Test; mMRC: modified Medical Research Council.
With regards to potential misdiagnosis of COPD, 160 individuals with a GP COPD code did not have airflow obstruction on LHC spirometry, whereas 676 had airflow obstruction and GP COPD code. The demography of these two groups were similar (no significant differences in age, deprivation or smoking status), although those without airflow obstruction had a higher body mass index (BMI) (mean±sd 31.2±6.1 kg·m−2 versus 27.6±5.8 kg·m−2, p<0.001). Symptom profiles and COPD CAT scores were similar between the two groups (e.g. score <10 in 30% versus 33%), but those without airflow obstruction had slightly worse mMRC dyspnoea score (p=0.043) and worse WHO performance status (p=0.024). Of note, the proportion of participants with LDCT evidence of emphysema (mild/moderate/severe/very severe) was 65% in those with known confirmed COPD, compared to 40% in those with possible misdiagnosed COPD, and 24% in the group without airflow obstruction or a COPD diagnosis. Multivariable logistic regression was undertaken to identify any determinants of a lack of airflow obstruction in those with a GP COPD diagnosis (table 2). The only parameter of significance was BMI, such that a higher BMI was associated with an absence of airflow obstruction.
TABLE 2.
Multivariable logistic regression for factors predicting an absence of airflow obstruction among all those with a general practice COPD diagnostic code (160 out of 836).
| OR (95% CI) | p-value | |
| Age | 0.99 (0.97–1.02) | 0.71 |
| Sex (male) | 0.97 (0.68–1.39) | 0.87 |
| BMI | 1.10 (1.07–1.14) | <0.001 |
BMI: body mass index.
Participants with known confirmed COPD and possible undiagnosed COPD were categorised according to GOLD classifications (table 3). Participants with known confirmed COPD were more likely to fulfil criteria for more severe disease, with 18% in GOLD stages 3/4 and 23% in GOLD group D compared to 4% and 5%, respectively, for those with possible undiagnosed COPD.
TABLE 3.
Categorisation of individuals in groups 1 and 2 according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) classifications.
| GP COPD code, airflow obstruction | No GP COPD code, airflow obstruction, COPD symptoms | |
| Participants | 676 | 655 |
| GOLD 1 | 204 (30.2) | 335 (51.1) |
| GOLD 2 | 351 (51.9) | 289 (44.1) |
| GOLD 3 | 104 (15.4) | 28 (4.3) |
| GOLD 4 | 17 (2.5) | 3 (0.5) |
| Missing data | 0 (0) | 0 (0) |
| GOLD group A | 193 (28.6) | 317 (48.4) |
| GOLD group B | 292 (43.2) | 286 (43.7) |
| GOLD group C | 29 (4.3) | 12 (1.8) |
| GOLD group D | 158 (23.4) | 34 (5.2) |
| Missing data | 4 (0.6) | 6 (0.9) |
Data are presented as n or n (%). GP: general practitioner; GOLD 1: forced expiratory volume in 1 s (FEV1) ≥80% pred; GOLD 2: 50%≤FEV1<80% pred; GOLD 3: 30%≤FEV1<50% pred; GOLD 4: FEV1 <30% pred; GOLD A: low symptoms, low exacerbations; GOLD B: high symptoms, low exacerbations; GOLD C: low symptoms, high exacerbations; GOLD D: high symptoms, high exacerbations.
Discussion
In this analysis of spirometry performed in a population at increased risk of lung cancer attending for lung cancer screening, a high prevalence of unexplained airflow obstruction was found. One in six (655 out of 3920; 17%) individuals attending for a LHC and undergoing spirometry was found to have possible undiagnosed COPD, and of all participants with symptomatic airflow obstruction (excluding those with asthma), less than half had a GP COPD diagnostic code (49%; 620 out of 1275). Furthermore, of the 836 participants with a GP COPD diagnostic code, 19% (n=160) did not have airflow obstruction on LHC spirometry, suggesting potential misdiagnosis.
Possible undiagnosed COPD
Overall, participants with possible undiagnosed COPD had less severe symptoms, less impaired lung function and less evidence of emphysema on CT than those with a GP COPD code and airflow obstruction, and were classified into less severe GOLD groups accordingly. This is partly to be expected, as those with a greater symptom burden are more likely to seek healthcare. In both our comparative and multivariable logistic regression analyses, those with possible undiagnosed COPD were more likely to be male and currently smoke. This is similar to findings in large population-based studies. An analysis of populations undergoing spirometry across 27 nations found male sex, lack of respiratory symptoms and less severe airflow obstruction to be positively associated with COPD underdiagnosis in multivariate analysis [2]. Similarly, in multivariate analyses of three national surveys in the United States [3] and a Canadian cohort [17], undiagnosed COPD was consistently associated with better overall health status, fewer respiratory symptoms and better lung function compared to those with diagnosed COPD. However, even though undiagnosed patients may have milder disease, this may still be prognostically significant, with increased risk of exacerbations, pneumonia and death reported in this population [4]. The association between current smoking status and an absence of a COPD diagnosis was seen in two studies [2, 4], but not in others [3, 17].
Possible misdiagnosed COPD
Of those participants with a GP COPD code, 19% did not have airflow obstruction and may have misdiagnosed COPD, although interpretation of this finding is qualified by some study limitations, as outlined later. Notwithstanding these limitations, the symptom burden and overall quality-of-life measures were similar between those with potential misdiagnosed COPD and those with confirmed COPD (i.e. GP code and airflow obstruction). Similar rates of inhaler use (85% versus 90%), antibiotic prescription in primary care (38% versus 45%) and hospital admission for respiratory problems (7% versus 6%) were reported by these two groups (supplementary tables E2 and E4). The only factor that was predictive of misdiagnosed COPD among all those with a GP COPD code was a raised BMI. We were not able to review previous primary care records for these participants, so are unable to comment on the basis on which the previous diagnosis of COPD was made. Possible explanations could be that this diagnostic code was added to their primary care record in the absence of spirometry measurement, following spirometry which was incorrectly interpreted as showing airflow obstruction, or that this diagnosis was appropriately made (i.e. previous post-bronchodilator spirometry confirmed airflow obstruction), but this was not replicated in spirometry measured during the LHC for some reason.
Misdiagnosis of COPD is also well-reported in other studies (sometimes referred to as overdiagnosis). Analysis of 23 populations across 20 countries participating in the Burden of Obstructive Lung Disease (BOLD) study [18] showed that the majority of participants with a medical diagnosis of COPD did not have airflow obstruction. Comparing false positive cases with true positive cases, rates of symptoms and quality of life scores were similar, but the false positive cases were more likely to be overweight or obese compared to those with correct diagnoses, matching the findings presented here. Cohorts of current or ex-smokers with significant respiratory symptoms and impairments without demonstrable airflow obstruction have been well described in other large series [19, 20]. The prevalence and severity of emphysema on CT reported here was higher for participants with potential misdiagnosed COPD (40%) compared to those with no COPD code or airflow obstruction (24%), indicating greater objective smoking-related pathology even in the absence of airflow obstruction. A proportion of people in this population may be labelled as having “pre-COPD”, as described in the latest GOLD report [7]. However, the extensive use of inhaled medications in this population is not evidence-based, and for some participants may be causing harm. A recent randomised trial of inhaled dual bronchodilator therapy showed no benefit in symptomatic tobacco-exposed people with preserved lung function as assessed by spirometry [21]. In addition, nearly half the population reported here were taking regular inhaled corticosteroids, which have been associated with a higher risk of pneumonia [22].
Comparison to other LHC programmes
The finding in this cohort of 17% of participants having possible undiagnosed symptomatic COPD is broadly similar to results described in other LHC programmes in the UK. The proportions of LHC attendees with possible undiagnosed symptomatic COPD were 10% (250 out of 2525) in the Manchester Lung Health Check programme [23], 19% (190 out of 986) in the Lung Screen Uptake Trial (LSUT) [24] and 20% (3154 out of 16 010) in the SUMMIT Study [25]. As in our analysis, those with possible undiagnosed COPD tended to have milder symptoms and better lung function than individuals with diagnosed COPD. Thus the proportion of undiagnosed patients with an MRC dyspnoea score ≥2 was 4.5% in Manchester and 3.4% in LSUT. The mean FEV1 expressed as percentage predicted value was 80% pred in the current study compared to 83% pred in Manchester, 79% pred in LSUT [24] and 68% pred in the SUMMIT Study [25]. None of the previous UK studies reported the proportion of participants with known COPD showing nonobstructed spirometry [23–25], so comparison of misdiagnosis is not possible. Only one study has looked at downstream consequences of suspected COPD diagnosis during lung cancer screening [26]. Of 55 patients referred to primary care from a lung screening pilot, only 51% attended their GP appointment, 38% were referred for community respiratory assessment, 11% commenced pharmacotherapy and 2% underwent pulmonary rehabilitation.
Strengths and limitations
This is one of the largest cohorts of participants in LHC programmes in which lung function data linked to demographic, clinical and imaging data are presented. In addition, this is the first study to use GP-coded diagnoses for COPD, and the first to assess possible misdiagnosed COPD detected during a LHC programme. There are a number of limitations of this analysis relating to lung function testing. This was performed pre-bronchodilator as standard; there was no record of whether participants on inhaled medication had taken therapies on the day of testing; and quality assurance data on variability (operator, technique or patient) are not available. The generally accepted definition of the diagnosis of COPD requires confirmation of airflow obstruction following administration of bronchodilator medication. We are therefore unable to comment on the proportion of those with possible undiagnosed COPD with confirmed disease based on this criterion. In addition, there are limitations with regards to primary care data. Previous diagnoses of asthma were based on self-report only, as codes relating to asthma were not extracted. Although COPD GP codes were extracted, further details (e.g. clinical symptoms and/or spirometry at time of diagnosis) were not.
Implications for practice
Many nations are currently considering nationwide implementation of LDCT screening for lung cancer. COPD and lung cancer share a common risk factor, so understandably there is interest in delivering spirometry alongside lung cancer screening. These data, in conjunction with those from other programmes, indicate that between 10% and 20% of those attending for screening may have undiagnosed symptomatic COPD, although these undiagnosed individuals tend to be less symptomatic with better preserved lung function. However, other than smoking cessation (which should be offered as standard to all screening participants who continue to smoke), there is limited evidence of improvement in clinical outcomes from interventions in minimally symptomatic patients. A recent review by the USPSTF concluded that there are still no comparative studies on the effectiveness of screening or active case-finding for COPD on patient health outcomes [27]. In addition, recently published diagnosis and treatment outcomes from a proportion of the participants reported here illustrate some of the “real-world” challenges in acting on spirometry results collected in this setting, including a reluctance of many participants to attend for further assessment [28]. Finally, there is a need to determine the impacts of offering spirometry alongside LDCT screening for the screening programme as a whole, for example measuring participation, adherence and participant satisfaction parameters.
Summary
Undiagnosed COPD is relatively common in LHC attendees, and delivery of spirometry alongside LDCT screening may provide an opportunity for earlier diagnosis in this population. However, further work is needed to address the challenges in acting on airflow obstruction detected in this context, including understanding why a proportion of participants decline further assessment. In addition, there is a lack of evidence describing the impact of spirometry delivered in this context on clinical outcomes which should be addressed in future studies. Separately, nearly one in five patients with a GP-coded diagnosis of COPD does not have airflow obstruction, and yet this group is highly symptomatic, has poor health status and is heavily treated with inhaled medications which do not have an evidence base. There is an urgent need to better clarify the aetiology of these patients’ respiratory symptoms to facilitate evidence based interventions that improve clinical outcomes.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00203-2023.SUPPLEMENT (566.6KB, pdf)
Acknowledgements
We acknowledge the contribution of the whole YLST clinical team (Sayyorakhon Alieva, Carol Bisby, Andy Cameron, Richard Cannon, Elly Charles, Suzette Colquhoun, Sam Curtis, Angie Dunne, Melanie Brear, Fazia Fazal, Helen Ford, Alice Forkin, Rita Haligah, Jade McAndrew, Sadia Moyudin, Joseph Peill, Angelika Pelka, Ellie Scott, Sophie Stevenson (Department of Research and Innovation, Leeds Teaching Hospitals, Leeds, UK) and Cat Bruckner and Matt Ward (Leeds Institute of Health Science, University of Leeds, Leeds, UK)).
Provenance: Submitted article, peer reviewed.
Support statement: This study was funded by Yorkshire Cancer Research (award reference L403). From September 2021, P. Alexandris was supported by the Barts Hospital Charity (MRC&U0036). P.A.J. Crosbie is supported by the Manchester National Institute for Health Research Manchester Biomedical Research Centre (IS-BRC-1215-20007). Funding information for this article has been deposited with the Crossref Funder Registry.
Statement on data sharing: In order to meet our ethical obligation to responsibly share data generated by clinical trials, YLST operates a transparent data sharing request process. Anonymous data will be available for request once the study has published the final proposed analyses. Researchers wishing to use the data will need to complete a Request for Data Sharing form describing a methodologically sound proposal. The form will need to include the objectives, what data are requested, timelines for use, intellectual property and publication rights, data release definition in the contract and participant informed consent etc. A Data Sharing Agreement from the sponsor may also be required.
Conflicts of interest: C. Bradley reports that the European Respiratory Society funded their attendance at the ERS International Congress in 2021 as they won a Best Abstract prize.
Conflicts of interest: P. Alexandris has nothing to disclose.
Conflicts of interest: D.R. Baldwin reports honoraria for presenting from MSD, Roche, BMS and AstraZeneca.
Conflicts of interest: R. Booton has nothing to disclose.
Conflicts of interest: M. Darby has nothing to disclose.
Conflicts of interest: C.J. Eckert has nothing to disclose.
Conflicts of interest: R. Gabe has nothing to disclose.
Conflicts of interest: N. Hancock has nothing to disclose.
Conflicts of interest: S. Janes reports consulting fees from AstraZeneca, Johnson and Johnson, Bard1 (consultancy) and Optellum (advisory board), honoraria from Chiesi for a lecture, and Takeda funded their attendance at conference.
Conflicts of interest: M. Kennedy has nothing to disclose.
Conflicts of interest: J. Lindop has nothing to disclose.
Conflicts of interest: R.D. Neal has nothing to disclose.
Conflicts of interest: S. Rogerson has nothing to disclose.
Conflicts of interest: B. Shinkins is a member of the the UK National Screening Committee (unpaid).
Conflicts of interest: I. Simmonds has nothing to disclose.
Conflicts of interest: S. Upperton has nothing to disclose.
Conflicts of interest: J. Vestbo reports payment from Boehringer Ingelheim to their hospital for an investigator-initiated clinical trial; consulting fees from AstraZeneca, ALK Abello, Boehringer Ingelheim, GSK, Novartis and TEVA; honoraria for presenting from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Novartis; and participant on data safety monitoring board or advisory board for Astra Zeneca and GSK.
Conflicts of interest: P.A.J. Crosbie reports consulting fees and stock options from Everest Detection, and honoraria from AstraZeneca, Novartis and North West eHealth.
Conflicts of interest: M.E.J. Callister has nothing to disclose.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. . Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128. doi: 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamprecht B, Soriano JB, Studnicka M, et al. . Determinants of underdiagnosis of COPD in national and international surveys. Chest 2015; 148: 971–985. doi: 10.1378/chest.14-2535 [DOI] [PubMed] [Google Scholar]
- 3.Martinez CH, Mannino DM, Jaimes FA, et al. . Undiagnosed obstructive lung disease in the United States. Associated factors and long-term mortality. Ann Am Thorac Soc 2015; 12: 1788–1795. doi: 10.1513/AnnalsATS.201506-388OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Çolak Y, Afzal S, Nordestgaard BG, et al. . Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med 2017; 5: 426–434. doi: 10.1016/S2213-2600(17)30119-4 [DOI] [PubMed] [Google Scholar]
- 5.UK National Screening Committee . Screening for Chronic Obstructive Pulmonary Disease (COPD) in the General Adult Population: External Review Against Programme Appraisal Criteria for the UK National Screening Committee. 2018; pp. 1–65. Available from: https://view-health-screening-recommendations.service.gov.uk/copd/.
- 6.Siu AL, Bibbins-Domingo K, Grossman DC, et al. . Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA 2016; 315: 1372–1377. doi: 10.1001/jama.2016.2638 [DOI] [PubMed] [Google Scholar]
- 7.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report). 2023. Available from: http://goldcopd.org/.
- 8.National Institute for Health and Clinical Excellence (NICE) . Chronic Obstructive Pulmonary Disease in over 16s: Diagnosis and Management. 2018. www.nice.org.uk/guidance/ng115. Date last updated: 26 July 2019.
- 9.Aberle, DR, Adams, AM, Berg, CD, et al. . Reduced lung cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365: 395–409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Koning HJ, van der Aalst CM, de Jong PA, et al. . Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020; 382: 503–513. doi: 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 11.UK National Screening Committee . Lung Cancer: UK NSC Screening Recommendation. 2022. https://view-health-screening-recommendations.service.gov.uk/lung-cancer/.
- 12.Crosbie PA, Balata H, Evison M, et al. . Implementing lung cancer screening: baseline results from a community-based ‘Lung Health Check’ pilot in deprived areas of Manchester. Thorax 2019; 74: 405–409. doi: 10.1136/thoraxjnl-2017-211377 [DOI] [PubMed] [Google Scholar]
- 13.Quaife SL, Ruparel M, Dickson JL, et al. . Lung Screen Uptake Trial (LSUT): randomized controlled clinical trial testing targeted invitation materials. Am J Respir Crit Care Med 2020; 201: 965–975. doi: 10.1164/rccm.201905-0946OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosbie PA, Gabe R, Simmonds I, et al. . Yorkshire Lung Screening Trial (YLST): protocol for a randomised controlled trial to evaluate invitation to community-based low-dose CT screening for lung cancer versus usual care in a targeted population at risk. BMJ Open 2020; 10: e037075. doi: 10.1136/bmjopen-2020-037075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sylvester KP, Clayton N, Cliff I, et al. . ARTP statement on pulmonary function testing 2020. BMJ Open Respir Res 2020; 7: e000664. doi: 10.1136/bmjresp-2020-000575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence (NICE) . Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care. 2010. www.nice.org.uk/guidance/cg101. Date last updated: 23 June 2010.
- 17.Labonté LE, Tan WC, Li PZ, et al. . Undiagnosed chronic obstructive pulmonary disease contributes to the burden of health care use. Data from the CanCOLD Study. Am J Respir Crit Care Med 2016; 194: 285–298. doi: 10.1164/rccm.201509-1795OC [DOI] [PubMed] [Google Scholar]
- 18.Sator L, Horner A, Studnicka M, et al. . Overdiagnosis of COPD in subjects with unobstructed spirometry: a BOLD analysis. Chest 2019; 156: 277–288. doi: 10.1016/j.chest.2019.01.015 [DOI] [PubMed] [Google Scholar]
- 19.Woodruff PG, Barr RG, Bleecker E, et al. . Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med 2016; 374: 1811–1821. doi: 10.1056/NEJMoa1505971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regan EA, Lynch DA, Curran-Everett D, et al. . Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med 2015; 175: 1539–1549. doi: 10.1001/jamainternmed.2015.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han MK, Ye W, Wang D, et al. . Bronchodilators in tobacco-exposed persons with symptoms and preserved lung function. N Engl J Med 2022; 387: 1173–1184. doi: 10.1056/NEJMoa2204752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drummond MB, Dasenbrook EC, Pitz MW, et al. . Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 2008; 300: 2407–2416. doi: 10.1001/jama.2008.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balata H, Harvey J, Barber PV, et al. . Spirometry performed as part of the Manchester community-based lung cancer screening programme detects a high prevalence of airflow obstruction in individuals without a prior diagnosis of COPD. Thorax 2020; 75: 655–660. doi: 10.1136/thoraxjnl-2019-213584 [DOI] [PubMed] [Google Scholar]
- 24.Ruparel M, Quaife SL, Dickson JL, et al. . Prevalence, symptom burden, and underdiagnosis of chronic obstructive pulmonary disease in a lung cancer screening cohort. Ann Am Thorac Soc 2020; 17: 869–878. doi: 10.1513/AnnalsATS.201911-857OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tisi S, Dickson JL, Horst C, et al. . Detection of COPD in the SUMMIT study lung cancer screening cohort using symptoms and spirometry. Eur Respir J 2022; 60: 2200795. DOI: 10.1183/13993003.00795-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartlett EC, Belsey J, Derbyshire J, et al. . Implications of incidental findings from lung screening for primary care: data from a UK pilot. NPJ Prim Care Respir Med 2021; 31: 36. doi: 10.1038/s41533-021-00246-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin JS, Webber EM, Thomas R. Screening for chronic obstructive pulmonary disease: a targeted evidence update for the U.S. Preventive Services Task Force. Rockville, Agency for Healthcare Research and Quality (US), 2022: 21-05287-EF-1. [PubMed] [Google Scholar]
- 28.Bradley C, Boland A, Clarke L, et al. . Diagnosis and treatment outcomes from prebronchodilator spirometry performed alongside lung cancer screening in a Lung Health Check programme. Thorax 2023; 78: 543–550. doi: 10.1136/thorax-2022-219683 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00203-2023.SUPPLEMENT (566.6KB, pdf)