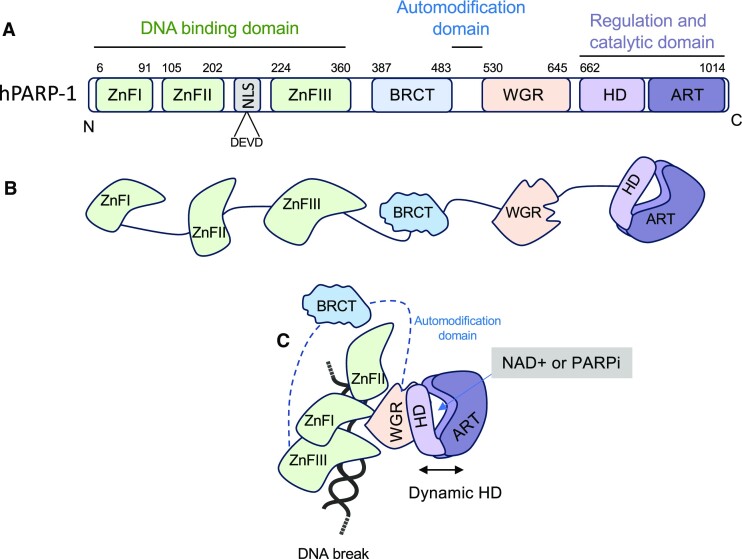
Figure 1.
(A) Human PARP-1 protein domains. PARP-1 bears six independently folded domains connected by flexible linker regions. The nucleic acid-binding region contains three zinc finger domains (ZnFI, ZnFII, ZnFIII, in green); a BRCA C-terminus (BRCT)-containing automodification domain (in blue); a nucleic acid-binding motif tryptophan– glycine–arginine (WGR, in orange) and the catalytic domain at the C-terminus, composed autoinhibitory helical domain (HD, light purple) and the ADP-ribosyl transferase fold (ART, dark purple), allowing PARP-1 to convert NAD+ into poly(ADP-ribose). (B) Communication between domains is essential for its DNA damage-dependent catalytic activity (modified from reference (334)). PARP-1 binding to DNA damage leads to systematic domain rearrangements, and allosterically leads to a dynamic HD (shown by double arrows). The binding to NAD+ (or PARPi) requires an open HD conformation leading to increased interaction with DNA.