Table 2.
Representative NLRP3 inhibitors are in preclinical or early clinical stages reported from recently filed patents and articles.
| Inhibitor | MOAs | IC50 (IL-1β release)# | Preclinical disease models* | Development Stage |
|---|---|---|---|---|
CY-09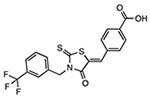
|
Binds to Walker A site in NACHT domain Inhibits ATPase activity |
6 μM | T2DM, diabetic retinopathy, diabetic liver injury, NAFLD, peritonitis, Muckle-Wells syndrome, epilepsy | Preclinical |
Tranilast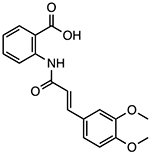
|
Disrupts NLRP3-NLRP3 oligomerization through NACHT domain binding | <25 μM | T2DM, NASH, colitis, peritonitis, Muckle-Wells syndrome, atherosclerosis | Phase II clinical trials to treat cryopin associated periodic syndrome (CAPS) (NCT03923140) Phase IV trial for percutaneous coronary intervention (NCT05130892) |
Oridonin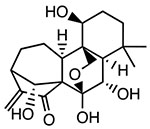
|
Covalently binds to Cys279 of NACHT domain and reduces NLRP3-NEK7 interaction | <0.5 μM | T2DM, liver fibrosis, IBD, peritonitis, hearing loss, pleurisy | Phase IV trial for percutaneous coronary intervention (NCT05130892) |
RRx-001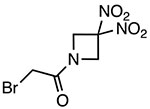
|
Covalently binds to Cys409 of NACHT domain and reduces NLRP3-NEK7 interaction | 117 nM | EAE, DSS colitis | Phase III clinical trials adjuvant treatment of small cell lung cancer with platinum (NCT03699956) |
Bay-11-7082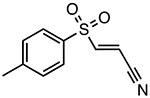
|
Inhibits ATPase activity Unknown binding site |
~3 μM | T2DM, IBD, psoriasis, lupus nephritis | Preclinical |
BOT-4-one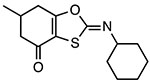
|
Inhibits ATPase activity Unknown binding site |
1.28 μM (LPS/ATP) 0.67 μM (LPS/nigericin) |
Peritonitis, dermatitis, psoriasis, arthritis | Preclinical |
OLT1177 (Dapansutrile)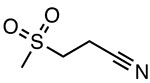
|
Inhibits ATPase activity Unknown binding site |
ND | AD, EAE, colitis, arthritis | Phase II clinical trials to treat acute gouty arthritis (EudraCT 2016-000943-14) |
INF39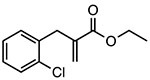
|
Covalent inhibitor of ATPase activity Unknown binding site |
~10 μM | IBD, colitis, pancreatitis | Preclinical |
JC-171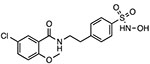
|
Reduces NLRP3-ASC interaction Unknown binding site |
8.45 μM | EAE | Preclinical |
| VTX2735 | Peripherally restricted NLRP3 inhibitor | 4 nM | N/A | Phase II clinical trial to treat cryopyrin associated periodic syndrome (NTC05812781) |
Emlenoflast (Inzomelid; MCC7840; IZD174)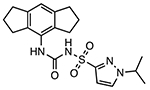
|
NLRP3 inflammasome inhibitor Unknown binding site |
4.7 nM | N/A | Phase I clinical trials for safety and tolerability (NCT04015076) Phase IIb clinical trials to treat cryopyrin associated periodic syndrome (CAPS) (EudraCT2020-000489-40) |
Selnoflast (RG6418; RO7486967)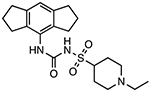
|
NLRP3 inflammasome inhibitor Unknown binding site |
≤ 1 μM (THP-1 cells) | N/A | Phase Ib clinical trials for safety and tolerability in ulcerative colitis patients (BP43099) Phase Ib clinical trials for safety and tolerability in patients with early idiopathic Parkinson’s disease (BP43176, CPMS 50823, IRAS 307220) |
IC50 values for IL-1β release inhibition in murine macrophage cells.
Abbreviations: AD: Alzheimer’s disease, PD: Parkinson’s disease, EAE: Experimental autoimmune encephalomyelitis, EAT: Experimental autoimmune thyroiditis, T2DM: Type 2 diabetes mellitus, NAFLD: Non-alcoholic fatty liver disease, NASH: Non-alcoholic steatohepatitis, IBD: Irritable bowel disease, DSS: Dextran sulfate sodium. ND: not determined.
