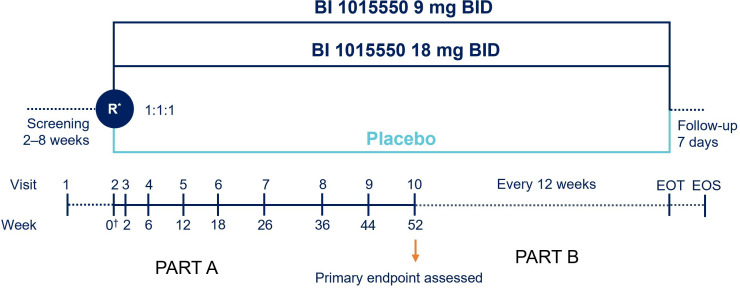
Figure 1.
FIBRONEER-IPF trial design. *Randomisation will be stratified by use of background antifibrotic (nintedanib/pirfenidone vs neither). †Day 1. Part B will begin from visit 10 to EOT over a variable period; patients will continue treatment with blinded trial medication and have trial visits every 12 weeks. EOS is expected to be up to 130 weeks with an assumed recruitment period of 18 months. BID, two times per day; EOS, end of study; EOT, end of trial (ie, last randomised patient reaches 52 weeks of treatment); R, randomisation.