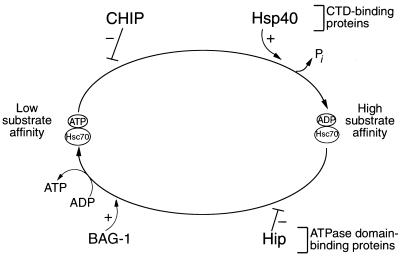
FIG. 10.
Model of the eukaryotic reaction cycle in the presence of CHIP, Hsp40, Hip, and BAG-1. The forward reaction, in which ATP is hydrolyzed to ADP and inorganic phosphate (Pi) is released, is enhanced by Hsp40. The biochemical data suggest that CHIP blocks this forward reaction. Hip stabilizes the ADP-bound, high-substrate-affinity conformation of Hsc70 and thus enhances chaperone activity. Conversely, BAG-1 accelerates nucleotide exchange, promoting substrate release and the formation of the low-substrate-affinity, ATP-bound conformation of Hsc70. In this model, both BAG-1 and CHIP would favor the low-affinity Hsc70 conformation, whereas Hip and Hsp40 would favor the high-affinity conformation. CTD, carboxy-terminal domain.