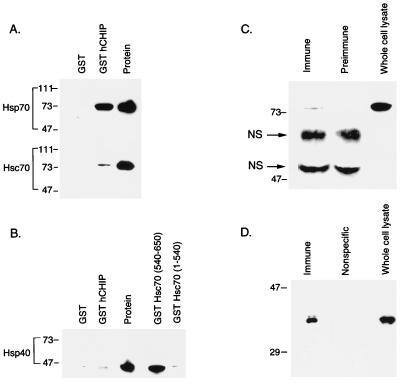
FIG. 5.
CHIP interacts with both Hsp70 and Hsc70 in vitro and in vivo. (A) Binding assays were performed with 1 μg of Hsp70 or Hsc70 plus 15 μg of GST or GST-hCHIP bound to glutathione-Sepharose 4B beads. Western blots were probed with an anti-Hsp70-Hsc70 monoclonal antibody. The protein lane contains 1 μg of the specific protein indicated at the left. Sizes are indicated in kilodaltons. (B) Binding assays were performed with 1 μg of Hsp40 plus 15 μg of GST, GST-hCHIP, GST-Hsc70(540-650), or GST-Hsc70(1-540). Western blots were probed with an anti-Hsp40 polyclonal antibody. The protein lane contains 1 μg of Hsp40. (C) Human skeletal muscle whole cell lysates were immunoprecipitated with rabbit immune serum to hCHIP or rabbit preimmune serum and analyzed by Western blotting with an anti-Hsp70-Hsc70 monoclonal antibody. Human skeletal muscle whole-cell lysates (50 μg) were loaded as a control. The two bands between 47 and 73 kDa are nonspecific (NS); the lower band is the heavy chain of IgG. (D) Human skeletal muscle whole-cell lysates were immunoprecipitated with an anti-Hsp70-Hsc70 monoclonal antibody or a monoclonal antibody recognizing nitric oxide synthase (Nonspecific) and analyzed by Western blot with rabbit anti-hCHIP serum. Whole-cell lysates (50 μg) were loaded as a control. The specific 35-kDa band seen in whole-cell lysates was detected only in immunoprecipitates obtained with the anti-Hsp70-Hsc70 antibody.