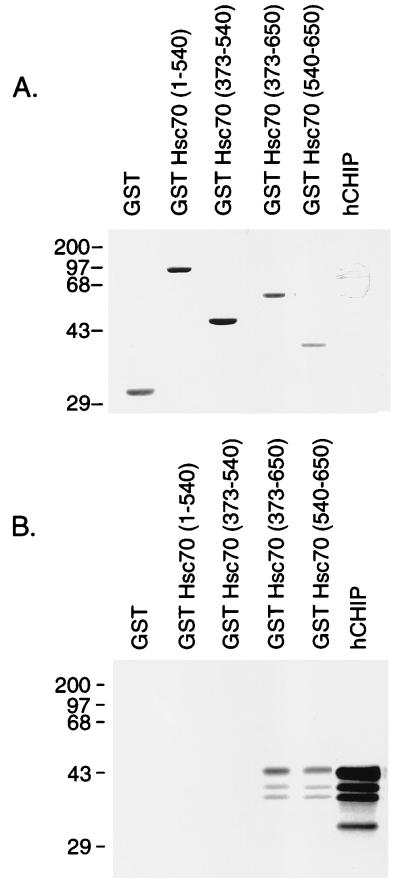
FIG. 6.
CHIP interacts with the carboxy-terminal domain of Hsc70 in vitro. Binding assays were performed with 3 μl of in vitro translation mixture containing 35S-labeled hCHIP with 3 μg of GST-Hsc70 deletion mutants. (A) Coomassie blue staining of the SDS-polyacrylamide gel demonstrates equivalent loading and appropriate folding of the fusion proteins. In vitro-translated 35S-labeled hCHIP is not visualized by Coomassie blue staining. Sizes are indicated in kilodaltons. (B) An autoradiogram of a duplicate gel shows that binding of the 35S-labeled hCHIP occurs to the carboxy-terminal GST-Hsc70 fusion proteins containing amino acids 373 to 650 and 540 to 650 of Hsc70 but not to those containing amino acids 1 to 540 or 373 to 540. An aliquot of 35S-labeled hCHIP was loaded into the last lane; lower-molecular weight species represent degradation of the in vitro-translated protein.