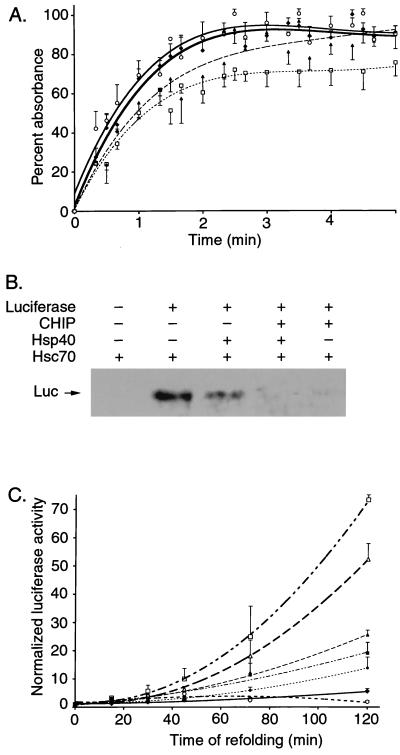
FIG. 9.
Effects of CHIP on chaperone functions of heat shock proteins. (A) The aggregation of rhodanese was measured at 340 nm over 5 min in the absence (⧫) or presence of CHIP (○), Hsp70-Hsp40 (□), or CHIP-Hsp70-Hsp40 (▴). The measured optical densities were normalized to the zero reading for each individual well, and the increase in absorbance was plotted as a percentage of the total increase of rhodanese alone. Each condition was repeated for a total of eight replicates, and points represent the mean ± standard error of the mean. (B) Binding assays were performed with denatured luciferase (Luc) and Hsc70 in the presence or absence of hCHIP and/or Hsp40. Binding of denatured luciferase was measured by coimmunoprecipitation using an anti-Hsc70 antibody, followed by blotting with an antiluciferase antibody. A representative experiment is shown. (C) Luciferase activity was measured as an indication of refolding after thermal denaturation. The refolding reactions were performed with Hsc70 (▵), Hsp40 (○), or both (□) in the absence (open symbols) and presence (closed symbols) of CHIP (⧫, CHIP alone). Luciferase activity was measured at various intervals between 0 and 120 min, and the activity for each reaction was normalized to luciferase in refolding buffer alone. Each condition was repeated for a total of 12 replicates, and points represent the mean ± standard error of the mean.