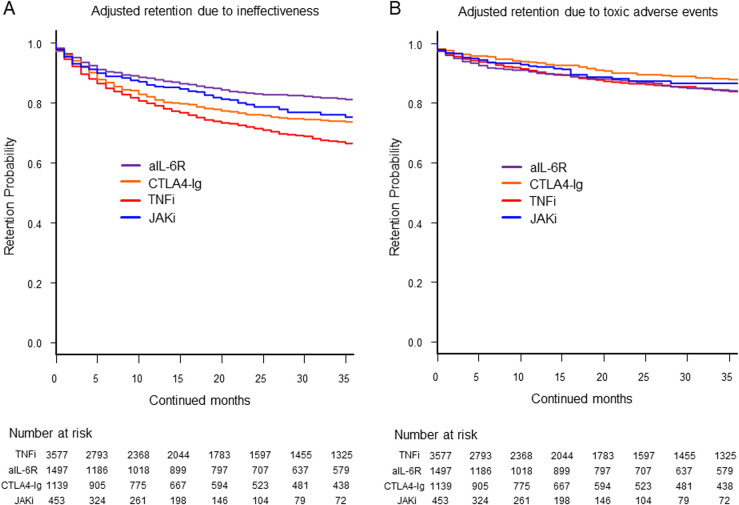
Figure 1.
Adjusted drug retention due to (A) ineffectiveness and (B) toxic adverse events. The adjusted confounders included baseline age, sex, disease duration, Clinical Disease Activity Index (CDAI), concomitant use of glucocorticoids, methotrexate and other conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), number of switched biological DMARDs or JAKis and prior use of TNFi, aIL-6R, CTLA4-Ig and JAKi. aIL-6R, anti-IL-6 receptor antibodies; CTLA4-Ig, cytotoxic T lymphocyte-associated antigen-4-Ig; JAKi, Janus kinase inhibitors; TNFi, tumour necrosis factor inhibitors.