Figure 3.
A single mutation of proline to leucine at amino acid position 220 of human SLC22A10 significantly enhances the accumulation of [3H]-estradiol-17b-glucuronide in HEK293.
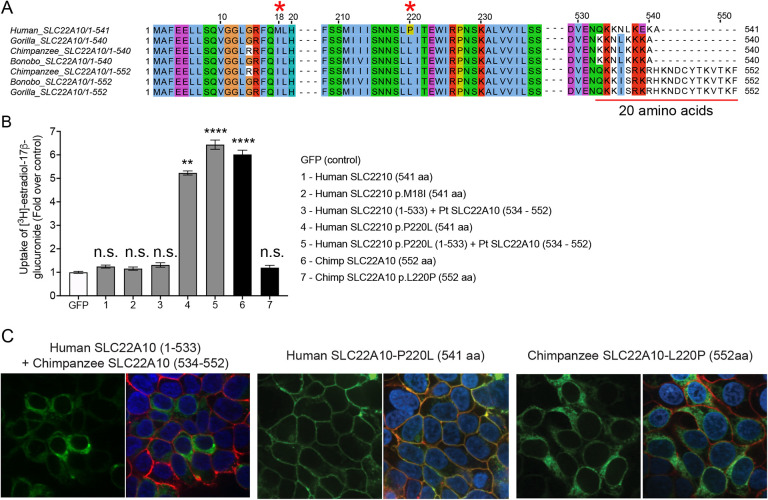
A. The amino acid sequence alignment of human SLC22A10 and SLC22A10 from other great apes (chimpanzee, bonobo and gorilla) shows that only the amino acids at positions 18 and 220 differ between the human ortholog and orthologs from great apes. Additionally, there are several amino acid differences starting at position 533.
B. The uptake of [3H]-estradiol-17b-glucuronide in HEK293 cells transiently transfected with plasmids encoding human SLC22A10 with reference amino acids or amino acids that are similar to those found in other great apes, namely SLC22A10-p.M18I and SLC22A10-p.P220L. A chimeric protein consisting of the first 533 amino acids of human SLC22A10 and the last 19 amino acids of chimpanzee SLC22A10 (534–552) was also evaluated, but did not significantly accumulate [3H]-estradiol-17b-glucuronide compared to the chimeric protein with p.P220L. The fold uptake of the substrate, relative to the control (GFP) cells, was plotted based on one representative experiment conducted in triplicate wells (mean ± s.d.). The statistical significance for cells transfected with SLC22A10 #4 (Human SLC2210 p.P220L (541 aa)), #5 (Human SLC2210 p.P220L (1–533) + Pt SLC22A10 (534 – 552)) and #6 (Chimp SLC2210 (552 aa)) is p<0.001.
C. This figure shows the plasma membrane localization of SLC22A10 conjugated to green fluorescent protein (GFP) in HEK293 cells. The GFP tag is located at the N-terminus of SLC22A10. Confocal imaging revealed that human SLC22A10-p.P220L localizes primarily to the plasma membrane of the cell, while there was no localization to the plasma membrane in cells expressing a chimeric protein or chimpanzee SLC22A10 with proline at the 220 amino acid position.