Abstract

The physical and chemical properties of atmospheric aerosol particles depend on their sources and lifetime in the atmosphere. In coastal regions, sources may include influences from marine, continental, anthropogenic, and natural emissions. In this study, particles in ten diameter-size ranges were collected, and particle number size distributions were measured, at Skidaway Island, GA in May and June 2018. Based on air mass back trajectories and concentrations of major ions in the particles, the air mass source regions were identified as Marine Influenced, Mixed, and Continental Influenced. Organic molecules were extracted from the particles using solid-phase extraction and characterized using tensiometry and high-resolution mass spectrometry. The presence of surfactants was confirmed in the extracts through the observation of significant surface tension depressions. The organic formulas contained high hydrogen-to-carbon (H/C) and low oxygen-to-carbon (O/C) ratios, similar to surfactants and lipid-like molecules. In the Marine Influenced particles, the fraction of formulas identified as surfactant-like was negatively correlated with minimum surface tensions; as the surfactant fraction increased, the surface tension decreased. Analyses of fatty acid compounds demonstrated that organic compounds extracted from the Marine Influenced particles had the highest carbon numbers (18), compared to those of the Mixed (15) and Continental Influenced (9) particles. This suggests that the fatty acids in the Continental Influenced particles may have been more aged in the atmosphere and undergone fragmentation. This is one of the first studies to measure the chemical and physical properties of surfactants in size-resolved particles from different air mass source regions.
Keywords: Atmospheric Aerosol Particles, Mass Spectrometry, Surfactants, Particle Composition
1.0. Introduction
The chemical composition of aerosol particles affects their ability to act as cloud condensation nuclei and form cloud droplets.1−3 The sources of particles can influence their composition and physical properties,4,5 thus modulating their direct and indirect influence on the climate. Understanding the chemical composition and physical properties of particles is important for accurately modeling both the impacts of the direct and indirect effects of particles on the current and future climate.6
While coastal regions may be influenced by air masses originating over the ocean, this is often not the only source of particles in these regions. Atmospheric aerosol particles in coastal regions consist of contributions from multiple sources, including marine, natural terrestrial, and anthropogenic sources.
Marine aerosol particles can be both primary and secondary in origin and vary in composition, accordingly. Primary marine aerosol particles are generated through bubble bursting at the sea surface.7 These particles are comprised of sea salt, with high concentrations of sodium and chloride, and ocean-derived organic compounds.8 The organic fraction of marine aerosol particles increases as particle size decreases and is more significant in submicron particles.9,10 The fraction of organic mass can change, depending on the biological activity of the water from which the particles are produced.8 Marine aerosol particles can be transported long distances and are often measured in high concentrations in coastal environments.11−13 Immediately upon emission, primary marine aerosol particles can participate in photochemical reactions, resulting in the fragmentation of large compounds to create volatile organic compounds (VOCs) and/or change chemical properties of the particles.14−16 Secondary marine aerosol particles are formed through the oxidation of gases emitted from the sea surface, as well as the oxidation of VOCs from primary marine aerosol particles.17,18 Non-sea salt sulfate can also be formed through the oxidation of dimethyl sulfide (DMS), emitted from the ocean, and contribute to a larger fraction of sulfate in marine aerosol.19,20
Coastal and estuarine plants can emit VOCs that form organic acids, including formate and acetate.21−23 Another major natural, terrestrial source of particles is mineral and soil dust, which can be identified through the presence of calcium and carbonate.24−26 The fraction of calcium in dust particles is much larger than that observed in sea spray particles.26−28 Dust has been observed as a major contributor to particle concentrations along the east coast of the United States.23,29,30
Anthropogenic aerosol particles can originate from a variety of sources and, thus, have diverse and complex compositions. Combustion emissions from biomass burning and fuel emissions have been linked to multiple inorganic ions, including sulfate, nitrate, and potassium in urban particles.31 Sulfate can also be produced through industrial methods and power generation.23,32
The different sources of aerosol particles, both natural and anthropogenic, result in a complex mixture of chemical and physical properties.33 High-resolution mass spectrometry provides insight into the complex mixture of particles through analysis of individual chemical formulas that can be categorized by hydrogen-to-carbon (H/C) and oxygen-to-carbon (O/C) ratios.34−38 This allows for broad classifications of organic molecules into different groups, such as aromatic compounds or fatty acids. Fatty acids are particularly of interest because of their surface-active properties and ability to act as surfactants.35,39 Fatty acids can originate from a variety of natural and anthropogenic sources, and their carbon chain lengths can vary, depending on sources and atmospheric processing.40
Surfactants are organic molecules that reduce the surface tension at the interface of a solution.41,42 Surfactants have been measured in seawater43−46 and in marine aerosol particles.47,48 Other studies have observed surfactants in anthropogenic-influenced particles,49,50 ambient urban particles,51 and biogenic terrestrial particles.39,52 Surfactants can impact particle hygroscopic growth53,54 and their potential to act as cloud condensation nuclei.55−57 Recent work has used high-resolution mass spectrometry to identify fatty acids and other surfactants in aerosol particles.34,35 These results can be combined with surface tension measurements to confirm the presence of surfactants.58
In this study, high-resolution mass spectrometry was used to identify and characterize surfactants extracted from size-separated aerosol particles collected from three different air mass source regions sampled from Skidaway Island, GA in May and June 2018. Particle number size distributions were collected simultaneously and varied in number concentrations, depending on the air mass source region. Inorganic ions measured with ion chromatography and air mass back trajectories confirmed three different air mass source regions. Here, the presence of surfactants in these particles was confirmed using tensiometry, chemical formulas were identified as surfactant-like using high-resolution mass spectrometry, and the chain lengths of fatty acids are reported for the samples from the different air mass source regions. The goal of this work is to determine the mass spectral signatures and sources of surfactants in size-resolved coastal aerosol particles and demonstrate the presence of surfactants in coastal aerosol particles from both marine and nonmarine sources.
2.0. Method
2.1. Particle Sample Collection
Aerosol particle samples were collected at Skidaway Institute of Oceanography (31.989584° N, 81.021622° W) outside of the Geochemistry Building at a height of ∼1.5 m. The sampling site is ∼35 m from the Skidaway River, ∼7 km from Wassaw Sound, and ∼11 km from the outer coast. Sampling was done over a two-week period from May 23, 2018 to June 5, 2018. However, there was no sample collection on May 27, 2018 due to the weather conditions, which included significant rainfall.
Size-fractionated particle samples were collected using two 8-stage Micro-Orifice Uniform Deposition Impactors without rotators (MOUDI, Model 100-NR, TSI, USA).59 Particles were collected on precombusted aluminum substrates for each stage of the MOUDI and a precombusted quartz fiber filter after the final stage. Samples were collected for 20–24 h at a flow rate of 30.0 L min–1. Incoming air was dried by using a diffusion dryer prior to sampling. After sample collection, the substrates were folded and placed in precleaned, individual 2-dram glass vials, wrapped in aluminum foil, and frozen until further analysis at the University of Georgia.
Three MOUDI sample time periods, including the samples from each of the 10 size fractions, were selected for analyses in this study. These samples were collected on May 26–27 (5/26/18 9:37 to 5/27/18 9:33), May 28–29 (5/28/18 10:44 to 5/29/18 9:50), and June 4–5 (6/4/18 10:24 to 6/5/18 7:20). As discussed in Section 3.0, these three time periods represent meteorological variability over the entire project. Each of the three sample time periods contains 10 individual samples, one in each particle size fraction (n = 30 total).
Field blanks were collected for the MOUDI samples on May 25 (5/25/18 9:32), May 28 (5/28/18 10:02), and June 3 (6/3/18 10:18). Substrates were loaded in the MOUDI, and it was connected in line for 10 s, without flow. The blanks from May 28 to June 3 were used in this study.
2.2. Particle Number Size Distributions
Particle number size distributions were measured using an aerodynamic particle sizer (APS, Model 3321, TSI, USA) for particles with aerodynamic diameters ranging from 0.5 to 20 μm in diameter, operating with scan times of 1 min. Submicron particle number size distributions were measured using a scanning electrical mobility spectrometer (SEMS, Model 2100, Brechtel, USA) for particles with mobility diameters ranging from 10 nm to 1 μm, operating with scan times of 50 s. The APS and SEMS instruments shared a single inlet containing an upstream diffusion drier, and the sample flow was split using a custom-made stainless-steel splitter. The silica gel in the dryer was changed daily to maintain a sample flow relative humidity of <70%.
The particle mobility diameters measured with the SEMS were converted to aerodynamic diameters using an assumed particle density of 2.12 g cm–1.60,61 The APS and SEMS number size distributions were averaged over the same 10 min intervals and then merged at an aerodynamic particle diameter of 932 nm, similar to previous work.60−62 An assumed particle density of 2.12 g cm–1 resulted in the best overlap between the SEMS and APS number size distributions for the three sample time periods. The number size distributions were used to calculate the total number and mass concentrations for each 10 min interval, as well as the particle masses in each of the MOUDI diameter ranges. The APS number size distributions for the June 4–5 sample were scaled to correct instrument error for low particle counts (see Text S1 in the Supporting Information).
2.3. HYSPLIT Back Trajectories
The Hybrid Single-Particle Lagrangian Integrated Trajectory model (HYSPLIT, NOAA) was used to calculate the back trajectories of the air masses and particles sampled during the campaign.63 Model back trajectories were created daily at altitudes of 500 m above the mean sea level and for a duration of 48 h, over the entire project. During individual MOUDI sampling times, 48 h model back trajectories were calculated every 12 h.
2.4. Particle and Organic Molecule Extraction
Water extractable species were extracted from the aluminum substrates and quartz fiber filters using a method of sonicating and/or vortexing, as described previously34,35,60,64−66 and briefly here. First, vials containing the substrates from the three selected samples were brought to room temperature, 5 mL of ultrapure water was added to each, and the vials were sealed. The substrates from the samples on May 26–27 and May 28–29 were agitated through two alternating cycles of vortexing and sonicating for 5 min each and subsequently transferred to individual precleaned 3-dram vials. This procedure was repeated a second time for these two samples, resulting in a total extract volume of 10 mL for each substrate. The substrates from the June 4–5 sample, with the addition of 5 mL of ultrapure water, were vortexed for 5 min, stored at 4 °C for 15 h, and were then vortexed again for 5 min. The solutions were transferred to individual precleaned 3-dram vials. Then, an additional 5 mL of ultrapure water was added to each substrate vial, followed by 5 min of vortexing and 30 min at rest, resulting in a total extract volume of 10 mL for each substrate. These two methods were assumed to extract organic species with similar efficiencies.
The 10 mL solutions, extracted from the substrates from all three MOUDI samples, were filtered using 0.45 μm poly(ether sulfone) membrane syringe filters into individual precleaned and preweighed 3-dram vials. All of the vials with the extract solutions were then weighed to obtain their exact masses.
The large organic compounds were then concentrated and separated from the rest of the sample matrix using a solid-phase extraction (SPE) method described by Burdette and Frossard.34 Briefly, the 10 mL extracts for each sample were processed through two SPE cartridges, ENVI-18 (C18 sorbent material, 0.5 g bed weight, MilliporeSigma, USA) and ENVI-Carb (graphitized carbon sorbent material, 0.5 g bed weight, MilliporeSigma, USA).
First, the ENVI-18 cartridges were conditioned with 6 mL of acetonitrile (MilliporeSigma, USA) and rinsed with 12 mL of ultrapure water. The 10 mL solutions from the initial sample extracts were pipetted into the cartridges and passed through the SPE material without vacuum. The eluates were collected in precleaned vials to save for extraction with the ENVI-Carb cartridge. The ENVI-Carb cartridges were conditioned with 6 mL of acetonitrile (MilliporeSigma, USA) and rinsed with 12 mL of ultrapure water. The 10 mL eluates from the ENVI-18 extractions were pipetted into the ENVI-Carb cartridges and passed through the SPE material without vacuum. The final eluates were collected in precleaned vials for ion chromatography analyses, as described in the next section.
For the elution of the organic molecules from the ENVI-18 and ENVI-Carb cartridges, the cartridges were rinsed using 12 mL of 0.1% triethylamine (MilliporeSigma, USA) in ultrapure water to remove any remaining salt in the SPE sorbent material.34 The cartridges were dried using vacuum, and the organic material was eluted in individual precleaned 1-dram vials using 4 mL of acetonitrile. The acetonitrile was evaporated using dry nitrogen gas (99.998% purity, Airgas, USA), and the vials with dried organic extracts were stored at 4 °C until analysis.
2.5. Measurements of Major Ions
The concentrations of major ions were measured using two Dionex Integrion high-pressure ion chromatography (HPIC, Thermo Fischer Scientific, USA) instruments. Samples and blanks were prepared by filling ion chromatography vials with 5 mL of the ∼10 mL final eluates from the solid-phase extractions. The autosampler split the volume of each sample between the two HPIC instruments, with one column to quantify the cations (IonPac CS12A, Thermo Fischer Scientific, USA) and the other to quantify the anions (IonPac AS18, Thermo Fischer Scientific, USA). Here, we measured the concentrations of cations lithium, sodium, ammonium, potassium, magnesium, and calcium and anions fluoride, acetate, formate, chloride, nitrite, carbonate, sulfate, and nitrate in each solution. The concentrations of the corresponding sample blanks were subtracted from the samples. Concentrations in solution were calculated from calibration curves for each ion of interest. Ion concentrations in particle samples were derived using the total volume of air sampled.
2.6. Surface Tension Measurements
The eluted and dried organic extracts from the ENVI-18 cartridges were rehydrated for surface tension analysis. Room-temperature organic extracts were dissolved in 40 μL of ultrapure water, and the surface tensions were measured using a pendent drop tensiometer (OCA 15EC, DataPhysics, Germany). The 40 μL solutions were suspended from syringes with 0.30 mm diameter needle tips for ∼30 s, at which point the surface tension was then measured continuously for ∼10 s to determine an average droplet surface tension. This process was repeated to obtain triplicate droplet measurements for each sample. The surface tension of the 40 μL hydrated extract is referred to herein as the surface tension minimum.34,43,58,65,66 Surface tension minimums are reported for the May 26–27 and May 28–29 samples. Surface tension minimums were not measured for the June 4–5 sample.
2.7. High-Resolution Mass Spectrometry
Organic fraction eluates were further hydrated and combined for analysis using high-resolution mass spectrometry. The dry ENVI-Carb extracts were rehydrated to 500 μL with a 1:1 mixture of methanol (ACS grade, Thermo Fisher Scientific, USA) and ultrapure water, followed by 5 min of sonicating and 30 s of vortexing. Then, the 500 μL the ENVI-Carb rehydrated extracts were transferred to the vials containing the 40 μL of hydrated ENVI-18 extracts for the corresponding samples. The combined extracts were then hydrated to final volumes of 1 mL, and an internal reference standard, Genistein (MilliporeSigma, USA), was added to a final concentration of 5 μM.
The combined, rehydrated samples and blanks were analyzed at the Proteomics and Mass Spectrometry Facility (PAMS) at the University of Georgia using an electrospray ionization quadrupole time-of-flight mass spectrometer (ESI-Q-TOF-MS; Impact II, Bruker, USA). This instrument has high mass accuracy and high resolution (∼20,000 resolving power at 400 Da), and all analyses were done using the negative ionization mode. A loop injection was used to introduce the blanks and samples to the mass spectrometer, and methanol was used as the carrier at a flow rate of 180 μL h–1. For each sample, an instrument run was started, where a blank corresponding to the selected sample was injected. Once the blank was finished, the sample was injected in the same run. After the sample was collected, a standard calibration mixture was injected. This process was repeated for each sample, and the instrument acquired mass spectra across a mass-to-charge (m/z) range of 50–1500 Da.
The mass spectra were initially processed using the instrument software (DataAnalysis, Bruker). The calibration was applied, and the mass accuracy of the Genistein was validated to ensure that the error was within 0.5 ppm. The mass spectral data was exported, and a custom MATLAB code was used to assign chemical formulas to the negative ion peaks in each sample spectrum, with a mass tolerance of 1.0 ppm.38,58 Chemical formulas were assigned with elemental ranges of C0–50, H0–100, O0–30, N0–6, and S0–2, following elemental constraints described by Stubbins et al.67 If multiple formulas were assigned to an individual peak, further analyses, including the Kendrick mass defect analysis, were used to determine a single, unambiguous formula.38,68,69 Only formulas that were unambiguously assigned to a specific peak were used in the analysis of the mass spectral data in this study. Formulas that contained both high O/C (oxygen to carbon, >0.6) and low H/C (hydrogen to carbon, <0.7) ratios were removed, since they were unlikely to be real organic formulas.38,67
2.8. Data Analyses and Statistical Tests
The programming language R was used for statistical analyses of aerosol particle chemical and physical properties as well as mass spectral data. Comparisons between sets of data were done using unpaired t-tests, and correlations were determined using Pearson correlation coefficient (R). Herein, any test with a p-value of <0.05 is labeled as “significant”.
3.0. Results and Discussion
3.1. Classification of Air Mass Source Regions
During the two-week sampling period, particle number concentrations increased with time (Figure 1). This general increase in number concentration corresponds to a shift in the local wind direction, which corresponded to changes in the origins and back trajectories of air masses that were sampled (Figures S1 and S2 in the Supporting Information). At the start of the sampling period, during the time frame of May 24–28, the back trajectories demonstrate that the air masses originated from southeast of the sampling site, over the Atlantic Ocean (Figure S1). This sampling period and the MOUDI sample collected therein (May 26–27) are defined as “Marine Influenced”, based on the air mass back trajectories originating from over the ocean and spending more than 75% of the time over the ocean prior to sampling (Figure S3 in the Supporting Information).16 However, this sampling period is not purely “clean marine” and may have additional influences from atmospheric aging and mixing with other sources.16 On May 28, 2018, the wind direction shifted, and the back trajectories show that the air masses originated from over Florida and from southwest of the sampling site, over the Gulf of Mexico (Figures S1 and S2 in the Supporting Information). This sampling period and the MOUDI sample collected therein (May 28–29), are defined as “Mixed” due to the overall mixture of air mass source regions (Figure S4 in the Supporting Information). On June 2, 2018, the back trajectories indicate that the air masses were traveling across the continental United States, originating northwest of the sampling site (Figure S2 in the Supporting Information). This sampling period and the MOUDI sample collected therein (June 4–5), are defined as “Continental Influenced”, since the air masses all originated from over the continental United States and may include a mixture of different continental sources, both natural and anthropogenic (Figure S5 in the Supporting Information).
Figure 1.
Number size distributions and total particle number concentrations (green) across the full sampling time for particles < 2 μm in diameter. White spaces indicate missing data or periods of instrument maintenance. Horizontal lines at the top indicate the three sampling periods investigated in this study, including Marine Influenced (blue), Mixed (green), and Continental Influenced (orange).
The number size distributions for the three selected MOUDI sample periods were isolated to compare their particle number and mass concentrations (Figure 2), with varying numbers of 10-min average particle number size distributions (132, 61, and 92 for the Marine Influenced, Mixed, and Continental Influenced sample periods, respectively). Marine Influenced particles have the lowest average dN/d(log Dp), compared to the other two sampling periods. Additionally, Marine Influenced particles show a bimodal distribution in the number size distributions, with the Aitken mode at 93 nm diameter and the accumulation mode at 272 nm, respectively (Figure 2). This bimodal peak shape in the number size distribution is unique to the Marine Influenced particles and is consistent with number size distributions of marine aerosol particles measured in previous studies.70-72 Similar distributions with Aitken and accumulation modes observed in marine aerosol particles have been attributed to non-precipitation cloud cycle processing.72,73
Figure 2.
(A, C, and E) Number and (B, D, and F) mass size distributions measured for the Marine Influenced samples (panels A and B), Mixed samples (panels C and D), and Continental Influenced samples (panels E and F). Thin lines represent individual 10 min averages (Marine Influenced: n = 132; Mixed: n = 61; and Continental Influenced: n = 92), and darker lines represent the average for the sampling period, for both the number and mass size distributions. Note that the y-axis ranges vary.
The number size distributions from the Mixed particles have a smaller average peak diameter of 62 nm as well as an overall number concentration higher than that of the Marine Influenced samples (Figure 2). The higher particle number concentration also corresponds to a higher particle mass during the Mixed sampling period compared to the Marine Influenced. Additionally, the Mixed number size distributions have a shoulder around 224 nm diameter but do not have the full second peak observed in the Marine Influenced (Figure 2). Single modes in number concentrations were previously observed in anthropogenic and continental influenced aerosol particles,71,72 consistent with the single modes observed here in the Mixed and Continental Influenced particle number size distributions.
The particle number concentrations were significantly higher in the Continental Influenced particles compared to the other two sampling periods (Figure 2). These number size distributions also had a larger diameter mode at ∼155 nm (Figure 2). The higher particle number concentration and larger average particle size contributed to a higher mass concentration for this sampling period (Figure 2).
The different shapes of particle number size distributions, as well as their peak locations, add confirmation that these three air masses were distinct from each other, resulting in different sources and probable variability in the fractions of primary and secondary (or chemically aged) particles in each population. The lower number concentrations and the distinct double peak in the number size distribution of the Marine Influenced sample indicates that these particles were primarily marine particles and had minor anthropogenic influences.71,72 The Continental Influenced number size distributions show a clear shift in the particle sizes to larger bins. This and the overall higher number concentrations may indicate particle growth in the atmosphere, contributing to the accumulation mode.71 Alternatively, the larger particle sizes could indicate the presence of dust from long-range transport.74
3.2. Major Ion Concentrations Vary with Particle Size and Source Regions
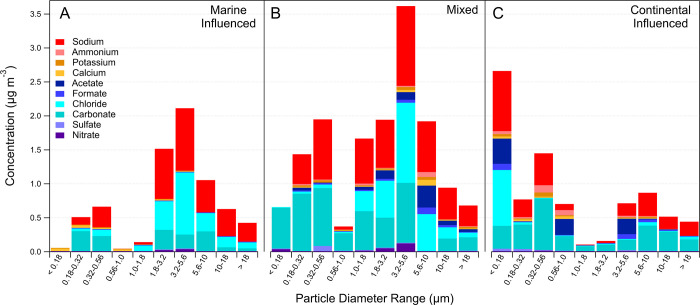
The differences in the particle number and mass distributions also correspond to variations in the major ion concentrations from the filters collected in each MOUDI diameter range (Figure 3 and Table S1 in the Supporting Information). HPIC has been used in prior research to identify and quantify the inorganic fraction of aerosol particles and infer particle sources.23,32,75,76
Figure 3.
Distribution of ion concentrations in the 10 different size bins collected for each sample, including (A) Marine Influenced, (B) Mixed, and (C) Continental Influenced air mass source regions. Ions with concentrations <0.02 μg m–3 were not included.
The major inorganic ions identified in the Marine Influenced particles were sodium, chloride, and carbonate (Figure 3a, n = 10). Sodium and chloride are commonly indicative of sea spray aerosol particles.23,77,78 The average fraction of chloride was the highest in the Marine Influenced particles compared to the Mixed and Continental Influenced particles. The ratio of mean sodium to mean chloride in the Marine Influenced particles is 1.6 (n = 10), and when compared to the ratio of sodium to chloride in seawater (0.56), demonstrates that these particles could be aged sea spray aerosol instead of freshly emitted.16,78 Atmospheric aging of sea spray aerosol particles causes a decrease in the fraction of chloride, and it is commonly replaced with non-sea salt sulfate78 or nitrate.79 However, in this study, sulfate was measured at low concentrations, around the detection limit, similar to those measured in the field blanks in the Marine Influenced samples. Additionally, the higher ratio of sodium to chloride in these samples also demonstrates that, while these samples are associated with air mass back trajectories from over the open ocean, they are not considered purely clean marine. While the source of carbonate in these samples has not been definitively identified, it is hypothesized that the small mass collected was slightly influenced by minor external mixing with a source of carbonate (such as soils and/or dust). The carbonate in the supermicron particles could also indicate a contribution of African dust, based on the path of the air mass back trajectories.74 Most of the measured ion mass is concentrated in the supermicron particles (Figure 3a) and is consistent with the calculated mass distributions (Figure 2b). The inorganic fraction accounted for ∼56% of the total mass calculated from the number size distributions, not taking into account potential sampling losses in the larger size fractions.
The Mixed particles also had high sodium, chloride, and carbonate concentrations, which varied across the different particle diameter ranges (Figure 3b, n = 10). The total mass concentrations of the major ions across all diameter ranges in this sample are higher than those of the Marine Influenced samples and include low concentrations of potassium and sulfate that were not detected in the Marine Influenced samples. These low concentrations of potassium and sulfate could indicate a minor influence of aged biomass burning and further demonstrate the mixed nature of this sample.80,81 While carbonate was present in some of the Marine Influenced samples, it is present in much larger concentrations in the Mixed particles (Figure 3). Carbonate is often indicative of mineral dust25,26 but can also be found in lake spray aerosol particles82 and Georgia estuarine water.83−85 Carbonate can also originate form cement dust at construction sites.86 The largest relative fraction of carbonate is in the submicron particles (Figure 3b). There is also a small fraction of calcium in these particles, which could also be another indicator of mineral dust.24,25,87
The supermicron particles in the Mixed sample contained chloride, but the ratio of mean sodium to mean chloride in these particles (1.87) is higher than that of the Marine Influenced samples. This could be attributed to the collection of more coastal and terrestrial aerosol particles, as well as more aged particles.78,88,89 This could also suggest an additional source of chloride, such as biomass burning.90 The supermicron particles also contained fractions of acetate and formate (up to 13% and 4%, respectively, in the 5.6–10 μm diameter particles). The presence of organic ions including acetate and formate could indicate the terrestrial sources of these particles.22 The inorganic fraction accounted for ∼30% of the total mass calculated from the number size distributions, not taking into account potential sampling losses in the larger size fractions.
The Continental Influenced sample had high concentrations of sodium, acetate, and carbonate (Figure 3c, n = 10). Compared to the other samples, these particles had the lowest overall sodium and chloride concentrations, which was expected since the air mass back trajectories did not originate from over the ocean (Figure 3). The concentration of sodium is similar to that of carbonate across most of the particle size ranges, suggesting that both might come from similar mineral dust sources91−93 or contain coagulated dust and sea spray particles due to processing in the atmosphere. This sample had the highest overall concentrations of ammonium and sulfate, observed in the submicron particles, which could be associated with industry and power generation.23,32 Additionally, the Continental Influenced sample had the largest overall fraction of the organic ion acetate, which may be associated with other terrestrial sources, such as vegetation and soil.94,95 This sample also had the highest overall mass concentration calculated from the number size distributions (Figure 2f) but not the highest concentration of major ions. The total mass of the major ions, including the inorganic fraction and the measured organic acids, accounts for ∼31% of the total calculated mass from the size distributions, excluding any sampling losses in the larger size fractions. This indicates that a significant fraction of the collected mass is likely organic matter, some of which will be discussed in the next section.
The Marine Influenced particles have more mass in the supermicron particles, while the Mixed particles have a similar distribution of masses in submicron and supermicron particles. The Continental Influenced particles have more mass in the submicron particles, compared to the supermicron particles. Together, the HYSPLIT analyses, the variation in the particle mass distributions, and the varying ion distributions confirm the three distinct sources of particles sampled during the Marine Influenced, Mixed, and Continental Influenced time periods.
3.3. Mass Spectral Comparison of Organic Fraction of Particles
The extracted organic fraction for each of the particle samples in each size fraction was analyzed using high-resolution mass spectrometry, and organic formulas were identified. In total, ∼7000 unique formulas were identified across the three particle sample periods (n = 30). The Marine Influenced samples had the highest total number of organic formulas identified (∼5000), followed by the Continental Influenced (∼3000) and Mixed (∼2000) samples.
The van Krevelen diagrams (Figure 4) show the relative H/C and O/C ratios of each chemical formula identified in the different samples, in the submicron and supermicron size ranges. The majority of formulas identified in the three samples, in both size ranges, have low O/C and high H/C values (Figure 4). The average values of H/C and O/C ratios were similar for each of the size bins across all three samples (Table 1), with generally high H/C (∼1.50) and low O/C (∼0.3), indicative of hydrocarbon-like molecules, as discussed later.
Figure 4.

Van Krevelen diagrams showing H/C and O/C for the organic formulas identified in the organic fraction of particles sampled from the Marine Influenced (A and B), Mixed (C and D), and Continental Influenced (E and F) samples. The organic formulas from the mass spectral data are separated by submicron (panels A, C, and E) and supermicron (panels B, D, and F) particle sizes.
Table 1. H/C, O/C, Percent Aliphatic (% Aliph.), Percent Aromatic (% Arom.), Percent Lipid-Like (% Lipid-Like), Percent Secondary Organic Aerosol Like (% SOA-Like), Percent Surfactant-Like (% Surf-Like), and Minimum Surface Tension (ST Min.) Measured for Organics Extracted from Particles in the Three Air Mass Source Regions, as a Function of Particle Diameter Range and for the Average (Avg.) of the Submicron and Supermicron Particle Diameters.
| <0.18 μm | 0.18–0.32 μm | 0.32–0.56 μm | 0.56–1.0 μm | 1.0–1.8 μm | 1.8–3.2 μm | 3.2–5.6 μm | 5.6–10 μm | 10–18 μm | >18 μm | submicron avg. | supermicron avg. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marine Influenced | ||||||||||||
| H/C | 1.48 | 1.40 | 1.44 | 1.44 | 1.39 | 1.44 | 1.38 | 1.46 | 1.39 | 1.41 | 1.44 | 1.41 |
| O/C | 0.31 | 0.32 | 0.31 | 0.30 | 0.31 | 0.32 | 0.32 | 0.33 | 0.31 | 0.33 | 0.31 | 0.31 |
| % aliph. | 36 | 35 | 34 | 38 | 31 | 37 | 34 | 35 | 29 | 46 | 36 | 35 |
| % arom. | 15 | 23 | 20 | 21 | 24 | 19 | 24 | 17 | 20 | 22 | 20 | 21 |
| % lipid-like | 30 | 28 | 29 | 29 | 28 | 29 | 27 | 28 | 24 | 26 | 29 | 27 |
| % SOA-like | 9 | 9 | 10 | 8 | 7 | 10 | 7 | 9 | 11 | 8 | 9 | 9 |
| % surf-like | 68 | 64 | 65 | 68 | 68 | 62 | 66 | 62 | 63 | 63 | 66 | 64 |
| ST min. | 36.0 | 36.8 | 34.8 | 39.5 | 38.9 | 48.3 | 37.5 | 43.9 | 43.2 | 41.6 | 36.8 | 42.2 |
| Mixed | ||||||||||||
| H/C | 1.55 | 1.55 | 1.52 | 1.56 | 1.49 | 1.58 | 1.49 | 1.55 | 1.53 | 1.55 | 1.55 | 1.53 |
| O/C | 0.27 | 0.27 | 0.28 | 0.27 | 0.30 | 0.29 | 0.30 | 0.29 | 0.30 | 0.31 | 0.27 | 0.30 |
| % aliph. | 39 | 38 | 34 | 41 | 33 | 41 | 33 | 38 | 39 | 39 | 38 | 37 |
| % arom. | 11 | 10 | 12 | 9 | 16 | 9 | 16 | 11 | 12 | 10 | 10 | 12 |
| % lipid-like | 40 | 36 | 30 | 34 | 27 | 41 | 24 | 32 | 33 | 30 | 36 | 31 |
| % SOA-like | 8 | 8 | 9 | 6 | 10 | 7 | 8 | 10 | 8 | 6 | 8 | 8 |
| % surf-like | 74 | 69 | 69 | 73 | 71 | 72 | 68 | 69 | 69 | 66 | 72 | 69 |
| ST min. | 38.5 | 42.7 | 40.3 | 40.0 | 45.9 | 37.9 | 38.3 | 38.6 | 41.8 | 42.3 | 40.4 | 40.8 |
| Continental Influenced | ||||||||||||
| H/C | 1.53 | 1.49 | 1.47 | 1.48 | 1.49 | 1.45 | 1.50 | 1.46 | 1.43 | 1.47 | 1.49 | 1.47 |
| O/C | 0.32 | 0.31 | 0.37 | 0.29 | 0.30 | 0.32 | 0.27 | 0.34 | 0.34 | 0.33 | 0.32 | 0.32 |
| % aliph. | 38 | 37 | 33 | 37 | 36 | 29 | 36 | 35 | 29 | 33 | 36 | 33 |
| % arom. | 11 | 13 | 16 | 16 | 15 | 16 | 14 | 15 | 19 | 14 | 14 | 15 |
| % lipid-like | 26 | 23 | 16 | 28 | 22 | 21 | 31 | 23 | 17 | 21 | 23 | 23 |
| % SOA-like | 12 | 12 | 14 | 8 | 10 | 11 | 6 | 14 | 10 | 9 | 12 | 10 |
| % surf-like | 64 | 63 | 53 | 67 | 66 | 63 | 73 | 63 | 60 | 66 | 62 | 65 |
The Marine Influenced samples have the largest spreads in formulas, with formulas spanning H/C ranges of 0.25 to 2.5 (Figure 4). Some formulas have high O/C (>0.8) as well, consistent with previous work showing high O/C for organic mass associated with primary marine aerosol (>1.00 for atmospheric primary marine aerosol and >0.50 for mixed marine aerosol) which was not attributed to atmospheric aging.16
Different regions of the van Krevelen diagrams and ranges of H/C and O/C can be used to broadly classify organic formulas.38,96 Secondary organic aerosol (SOA) has been associated with an H/C range of 1.0 to 1.75 and O/C range of 0.4 to 0.8.33,38,97 Here, the SOA region contains ∼9% of the assigned formulas in all three of the samples. The SOA-like organic formulas were slightly more prevalent in the Continental Influenced samples (∼11%), compared to the other two samples (8% for Marine Influenced and 9% for Mixed). Along with the higher concentrations of formate and acetate, this could be indicative of more atmospheric aging in the Continental Influenced samples, compared to the other samples. However, the SOA-like formulas were a small fraction of the total formulas and were similar across the three sampled air mass source regions. SOA has been observed to be a large fraction of the total organic mass in the southeastern United States in the summer.98,99 In this study, large organic molecules were concentrated and extracted for characterization using solid-phase extraction, which may exclude smaller SOA molecules. Additionally, some SOA may have formulas that fall outside of the defined O/C and H/C region, which was defined based on laboratory studies.38,97
The percentage of molecular formulas that were identified as aliphatic and aromatic compounds varied for the three air mass regions. Aromatic formulas were identified as those with a calculated aromaticity index value of greater than 0.5.38,58,67,100,101 In total across all three samples, almost 17% of the organic formulas were classified as aromatic (Table 1). The organic formulas from the Marine Influenced sample had the highest average percentage of aromatic compounds (20%) out of the three samples (Table 1), which may be due to local and transported ship emissions.33 This is demonstrated by the distribution of organic formulas in the van Krevelen diagrams, which shows a higher number of formulas with low H/C for the Marine Influenced particles, compared to the other samples (Figure 4).
Formulas were identified as aliphatic molecules if they contained an H/C ratio of >1.0 and a double bond equivalent (DBE)-to-carbon ratio of <0.3.67 Across all three samples, almost 36% of the assigned formulas were classified as aliphatic, with little variability in aliphatic fraction between the three samples, each averaging between 34% and 38% (Table 1). However, there are some minor differences when comparing the fractions of aliphatic formulas in different diameter size ranges. For both the Marine Influenced and the Continental Influenced particles, the 10–18 μm diameter size range had two of the lowest percentages of aliphatic compounds (Table 1), suggesting that there are fewer aliphatic compounds in the larger particles. The overall lowest percentage of aliphatic compounds was in the 1.8–3.2 μm diameter size range of the Continental Influenced sample (Table 1). The other two samples, Marine Influenced and Mixed, had above average percentages of aliphatic formulas in their 1.8–3.2 μm size bins. The differences in aliphatic fractions across the size bins for the different sample types are consistent with a different organic mass composition in the Continental Influenced samples, compared to the other samples.
For the samples from all three air mass source regions, the average percentage of aliphatic formulas is higher in the submicron particles than in the supermicron particles, with the Continental Influenced sample having the biggest difference (36%, n = 4; and 33%, n = 6, respectively), as shown in Table 1. The opposite trend is observed for aromatic assigned formulas, with a slightly higher percentage of aromatic organic compounds in the supermicron particles than in the submicron particles for all samples. The mass spectral characterization shows differences in the organic composition of identified chemical formulas across different diameter size ranges and particle source regions.
3.4. Surfactant Fraction of Organic Mass in Particles
3.4.1. Surfactants and Lipid-Like Organic Molecules in Particles
The presence of surfactant molecules in the organic fraction extracted from the particles was confirmed through surface tension measurements. The surface tension minimums (surface tensions of the organic extracts rehydrated in 40 μL of ultrapure water) are shown in Table 1 for the Marine Influenced sample and the Mixed sample. The minimum surface tensions are significantly depressed compared to a surface tension of pure water (72 mN m–1), across all of the particle size bins and in both sample types (n = 20), indicating the presence of surfactants.
The minimum surface tension is significantly lower for submicron particles than supermicron particles (unpaired Student’s t-test, p < 0.05), with mean values of 36.8 mN m–1 (n = 4) and 42.2 mN m–1 (n = 6), respectively, in the Marine Influenced sample (see Table 1, as well as Figure S6 in the Supporting Information). This indicates that submicron particles had higher concentrations of surfactants, or stronger surfactants, which had a large effect on the surface tension. This trend was not observed in the Mixed sample where the surface tension minimums were similar at 40.4 mN m–1 (n = 4) and 40.8 mN m–1 (n = 6) for the submicron and supermicron particles, respectively. This trend confirms that the influence of surfactants in the Mixed sample was different from that of the Marine Influenced sample, based on the source region of the air mass.
The van Krevelen diagrams were also used to broadly classify lipid-like formulas,38,96 which have previously been identified to be surfactant-like. Lipids generally have formulas with high H/C (>1.5) and low O/C (<0.3) (see Figure 4, as well as Figure S7 in the Supporting Information). Over 27% of the chemical formulas across all three samples and size ranges were identified as lipid-like (n = 30). The highest percentage of lipid-like formulas identified was in the Mixed particles (Table 1). The Continental Influenced samples had the highest percentage, ∼31%, of formulas that had low O/C (<0.3) and lower H/C (<1.5), identified as hydrocarbon-like.
To expand the classification and identify formulas that may be surfactant-like, in addition to the lipid-like formulas, the H/C and O/C of known surfactant standards from a vendor database were calculated (n ≈ 2400). A van Krevelen diagram was plotted to visualize the distribution of these surfactant formulas (see Text S2 and Figure S7 in the Supporting Information). More than 73% of the surfactant standards contained H/C ratios >0.4 and O/C ratios <0.35, and this region was thus classified as “surfactant-like” (Figure S7 in the Supporting Information). Previous work has also identified surfactants with similar H/C and O/C formulas.34,35,58
Using this classification for surfactant-like organic molecules, more than 60% of formulas identified in the Marine Influenced samples were classified as surfactant-like. A negative correlation (R = −0.68, n = 10) was observed between the percentage of formulas in the surfactant region and the minimum surface tension of the Marine Influenced samples for the different particle size ranges (Figure 5). This negative correlation demonstrates that this mass spectral classification identified the surfactant-like formulas that have the most influence on the surface tension of the Marine Influenced samples. Here, it is generally observed that the organic extracts from the submicron particles have a larger fraction of surfactant-like compounds and a lower minimum surface tension than those from the supermicron particles (Table 1, Figure 5). This is also consistent with previous work that observed a larger enrichment of surfactant fluorescent organic matter in generated sea spray aerosol as the particle size decreased.102 Previous work on marine aerosol observed a larger fraction of water-soluble organic compounds in fine particles, compared to coarse particles, and saw that the average surface tension of the particle extracts decreased as a function of water-soluble organic carbon concentration, inferring that the aliphatic and humic-like composition would result in surface-active properties.8,103 A correlation of this strength is not observed with the Mixed samples (see Figure S8 in the Supporting Information, n = 10), indicating that the identified formulas may be more variable in composition and strength in the Mixed sample across the different size ranges.
Figure 5.

Correlation between the percentage of organic formulas with H/C and O/C values in the surfactant-like range and the associated surface tension minimums for the Marine Influenced samples (n = 10). Markers are colored by supermicron (dark blue) or submicron (light blue) particle size ranges.
3.4.2. Fatty Acid Chain Length Varies with Air Mass Source Region
Sources of surfactants can differ for each sample air mass region. The Marine Influenced particles likely had surfactants from seawater that aggregate toward the sea surface and are aerosolized in the wave breaking and bubble bursting process. Seawater can be a source of different types of surfactants, including fatty acids, which have previously been characterized in particles produced by replicating the open ocean bubble bursting process.35 Fatty acids can also be emitted from terrestrial sources, such as trees and other vegetation,104 and may contribute significantly to surfactant concentrations in particles.40 Fatty acids are also emitted during biomass burning and during coal burning.37,105
Chemical formulas from high-resolution mass spectrometry analyses of the samples from the three source regions were compared to a list of identified molecules from Cochran et al.35 that contained saturated and unsaturated fatty acids, saturated oxo-fatty acids, saturated hydroxyl-fatty acids, saturated dicarboxylic acids, sulfates, and linear alkylbenzenesulfonates. In the particles from the three source regions, the most commonly observed molecules were tetradecanedioic, pentadecadienoic, and oxooctadecanoic acid. The unsaturated fatty acids, such as pentadecadienoic acid, were mainly found in the Mixed and Continental Influenced samples and were often found in both submicron and supermicron particles. Unsaturated fatty acids are often associated with short lifetimes due to the reactivity of the molecules,106,107 so this indicates that these fatty acids were likely from nearby terrestrial or anthropogenic sources.
The distribution of the identified fatty acid formulas in each sample, as a function of the number of carbon atoms in the formulas, is shown in Figure 6. The fatty acids from the Marine Influenced particles (n = 10) contained an average of 15.8 carbons, which is consistent with previous studies of fatty acids in marine aerosol particles.35,40,43 The Mixed samples (n = 10) had an average of 18.2 carbons per formula, and the Continental Influenced samples (n = 10) had 13.0. The low average number of carbons in fatty acids for the Continental Influenced samples is unexpected for biomass burning or fossil fuel producing particles and is likely mainly driven by other anthropogenic influences.40
Figure 6.

Number of occurrences of an identified fatty acid formula containing a specific number of carbon atoms for each sample. The average number of carbons for each sample is shown as a black marker.
Additionally, the most abundant fatty acid carbon chain lengths were compared for the three different samples (Figure 6). The most abundant fatty acid carbon chain length is 18 for the Marine Influenced sample, 15 for the Mixed sample, and 9 for the Continental Influenced sample. This further suggests that the Marine Influenced particles may contain fatty acids that were recently emitted from a marine source while the Continental Influenced particles may contain fatty acids from different sources, such as from plant waxes, or those that were fragmented due to photochemical aging in the atmosphere.40,108−112
4.0. Conclusion
This is one of the first studies to measure the organic composition of surfactant-like molecules in size-resolved atmospheric aerosol particles using high-resolution mass spectrometry. Large organic compounds were extracted from the particles collected in 10 size ranges by using solid-phase extraction. The concentrations of the major ions in particles combined with HYSPLIT analyses were used to identify samples originating from three distinct air mass source regions: Marine Influenced, Mixed, and Continental Influenced. The composition of the particles, including the major ions and organic fraction, varied with the air mass source region and particle size ranges.
Surfactant-like organic compounds were observed across all particle size ranges and in particles from all three source regions. Surface tension minimums, measured for the organic extracts, were significantly depressed (<45 mN m–1, n = 20) in all measured samples, confirming the presence of surfactant-like molecules. For the Marine Influenced sample, the surface tension minimum was lower in the submicron particles, compared to the supermicron particles, indicating stronger surfactants or higher concentrations of surfactants in the submicron Marine Influenced particles.
The van Krevelen diagrams show the formulas of many of the extracted organic compounds to be consistent with surfactants, with high H/C and low O/C values across all size bins and air mass source regions. Generally, the formulas assigned for organic molecules in particles in the submicron size range had higher H/C values than those in the supermicron particles for all sample sets. The percent aliphatic and percent aromatic were similar across size ranges for given sampling times. For the Marine Influenced particles, the fraction of formulas that fell in the H/C and O/C regions identified as surfactant-like had a negative correlation with surface tension minimums and comprised 61% of the total assigned formulas.
Formulas identified as fatty acids were observed in particles from all three of the source regions. The Marine Influenced sample had the highest abundance of longer chain fatty acids (C18), and the Continental Influenced sample had the highest abundance of shorter chain fatty acids (C9). This may indicate that the organic compounds in the Continental Influenced particles originated from different sources or were aged in the atmosphere through fragmentation of larger organic molecules compared to the organic compounds in the Marine Influenced particles. All measurements completed in this work confirm three distinct populations of particles exhibiting different compositions and relationships to surfactants found in the extracted organic fractions.
This exploratory work can be expanded to further investigate the influence of a larger range of particle sources on the composition of surfactants in atmospheric particles and to identify correlations between surface tension minimums and fraction of surfactant-like formulas across a wider range of particle sources and within a given size range. Ongoing work to measure the surfactant concentrations across the particle size ranges and comparison of the surfactant properties and formulas to the total concentrations are essential to broadening the understanding of the sources of this class of molecules and their role in particle formation and physiochemical characteristics.
Acknowledgments
Primary financial support for this work was provided by the University of Georgia Investment in Sciences Initiative and Office of Research, as well as the National Science Foundation (NSF) through an award to the University of Georgia (No. AGS-2239105). Funding for high-resolution mass spectrometry analyses was provided by the University of Georgia COVID Impact Research Recovery Funding (CIRRF) Program. The authors thank Dr. Dennis Phillips in the Proteomic and Mass Spectrometry (PAMS) facility at the University of Georgia for his assistance with mass spectrometry. The authors also thank the Director of Skidaway Institute of Oceanography, Professor Clark Alexander, for assistance in field study coordination and in sample collection. The authors thank Whitney Hudson for aiding in the collection of samples and Ariana Deegan for assisting with surface tension measurements.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsearthspacechem.3c00161.
Table showing the concentration of inorganic ions in each size bin measured using ion chromatography (Table S1); text explaining the scaling of the Continental Influenced APS number distributions (Text S1); figure showing the HYSPLIT back trajectories for each of the first 6 days (Figure S1); figure showing the HYSPLIT back trajectories for each of the last 7 days (Figure S2); figure showing the HYSPLIT back trajectories for the sample identified as from the Marine Influenced air mass source region (Figure S3); figure showing the HYSPLIT back trajectories for the sample identified as from the Mixed air mass source region (Figure S4); figure showing the HYSPLIT back trajectories for the sample identified as from the Continental Influenced air mass source region (Figure S5); figure showing the surface tension at each particle size for the Marine Influenced sample (Figure S6); text explaining the use of a vendor database to create a van Krevelen for surfactant standards (Text S2); figure showing the van Krevelen diagram for surfactant standards (Figure S7); figure showing correlation between surfactant-like formula percentage and surface tension for the Mixed samples (Figure S8) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Novakov T.; Penner J. E. Large contribution of organic aerosols to cloud-condensation-nuclei concentrations. Nature 1993, 365 (6449), 823–826. 10.1038/365823a0. [DOI] [Google Scholar]
- Kohler H. The nucleus in and the growth of hygroscopic droplets. Trans. Faraday Soc. 1936, 32 (2), 1152–1161. 10.1039/TF9363201152. [DOI] [Google Scholar]
- Facchini M. C.; Mircea M.; Fuzzi S.; Charlson R. J. Cloud albedo enhancement by surface-active organic solutes in growing droplets. Nature 1999, 401 (6750), 257–259. 10.1038/45758. [DOI] [Google Scholar]
- Pöschl U. Atmospheric Aerosols: Composition, Transformation, Climate and Health Effects. Angew. Chem., Int. Ed. 2005, 44 (46), 7520–7540. 10.1002/anie.200501122. [DOI] [PubMed] [Google Scholar]
- Jimenez J. L.; Canagaratna M. R.; Donahue N. M.; Prevot A. S. H.; Zhang Q.; Kroll J. H.; DeCarlo P. F.; Allan J. D.; Coe H.; Ng N. L.; et al. Evolution of Organic Aerosols in the Atmosphere. Science 2009, 326 (5959), 1525–1529. 10.1126/science.1180353. [DOI] [PubMed] [Google Scholar]
- Masson-Delmotte V.; Zhai P.; Pirani A.; Connors S. L.; Péan C.; Berger S.; Caud N.; Chen Y.; Goldfarb L.; Gomis M.. Climate change 2021: The physical science basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change 2021, 2. [Google Scholar]
- Blanchard D. C. Sea-to-air transport of surface active material. Science 1964, 146 (364), 396. 10.1126/science.146.3642.396. [DOI] [PubMed] [Google Scholar]
- O’Dowd C. D.; Facchini M. C.; Cavalli F.; Ceburnis D.; Mircea M.; Decesari S.; Fuzzi S.; Yoon Y. J.; Putaud J.-P. Biogenically driven organic contribution to marine aerosol. Nature 2004, 431, 676. 10.1038/nature02959. [DOI] [PubMed] [Google Scholar]
- Keene W. C.; Maring H.; Maben J. R.; Kieber D. J.; Pszenny A. A. P.; Dahl E. E.; Izaguirre M. A.; Davis A. J.; Long M. S.; Zhou X. L.; et al. Chemical and physical characteristics of nascent aerosols produced by bursting bubbles at a model air-sea interface. J. Geophys. Res.-Atmos. 2007, 112 (D21), 16. 10.1029/2007JD008464. [DOI] [Google Scholar]
- Quinn P. K.; Bates T. S.; Schulz K. S.; Coffman D. J.; Frossard A. A.; Russell L. M.; Keene W. C.; Kieber D. J. Contribution of sea surface carbon pool to organic matter enrichment in sea spray aerosol. Nat. Geosci. 2014, 7 (3), 228–232. 10.1038/ngeo2092. [DOI] [Google Scholar]
- Murphy D. M.; Anderson J. R.; Quinn P. K.; McInnes L. M.; Brechtel F. J.; Kreidenweis S. M.; Middlebrook A. M.; Pósfai M.; Thomson D. S.; Buseck P. R. Influence of sea-salt on aerosol radiative properties in the Southern Ocean marine boundary layer. Nature 1998, 392, 62. 10.1038/32138. [DOI] [Google Scholar]
- Jaeglé L.; Quinn P. K.; Bates T. S.; Alexander B.; Lin J. T. Global distribution of sea salt aerosols: new constraints from in situ and remote sensing observations. Atmos. Chem. Phys. 2011, 11 (7), 3137–3157. 10.5194/acp-11-3137-2011. [DOI] [Google Scholar]
- De Leeuw G.; Neele F. P.; Hill M.; Smith M. H.; Vignati E. Production of sea spray aerosol in the surf zone. J. Geophys. Res.: Atmos. 2000, 105 (D24), 29397–29409. 10.1029/2000JD900549. [DOI] [Google Scholar]
- Ciuraru R.; Fine L.; van Pinxteren M.; D’Anna B.; Herrmann H.; George C. Photosensitized production of functionalized and unsaturated organic compounds at the air-sea interface. Sci. Rep. 2015, 5, 10. 10.1038/srep12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert P. A.; Ciuraru R.; Rossignol S.; Passananti M.; Tinel L.; Perrier S.; Dupart Y.; Steimer S. S.; Ammann M.; Donaldson D. J.; et al. Fatty Acid Surfactant Photochemistry Results in New Particle Formation. Sci. Rep. 2017, 7, 11. 10.1038/s41598-017-12601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frossard A. A.; Russell L. M.; Burrows S. M.; Elliott S. M.; Bates T. S.; Quinn P. K. Sources and composition of submicron organic mass in marine aerosol particles. J. Geophys. Res.-Atmos. 2014, 119 (22), 12977–13003. 10.1002/2014JD021913. [DOI] [Google Scholar]
- Kroll J. H.; Seinfeld J. H. Chemistry of secondary organic aerosol: Formation and evolution of low-volatility organics in the atmosphere. Atmos. Environ. 2008, 42 (16), 3593–3624. 10.1016/j.atmosenv.2008.01.003. [DOI] [Google Scholar]
- Bertram T. H.; Cochran R. E.; Grassian V. H.; Stone E. A. Sea spray aerosol chemical composition: elemental and molecular mimics for laboratory studies of heterogeneous and multiphase reactions. Chem. Soc. Rev. 2018, 47 (7), 2374–2400. 10.1039/C7CS00008A. [DOI] [PubMed] [Google Scholar]
- Fung K. M.; Heald C. L.; Kroll J. H.; Wang S.; Jo D. S.; Gettelman A.; Lu Z.; Liu X.; Zaveri R. A.; Apel E. C.; et al. Exploring dimethyl sulfide (DMS) oxidation and implications for global aerosol radiative forcing. Atmos. Chem. Phys. 2022, 22 (2), 1549–1573. 10.5194/acp-22-1549-2022. [DOI] [Google Scholar]
- Fitzgerald J. W. Marine aerosols: A review. Atmos. Environ., Part A. Gen. Topics 1991, 25 (3), 533–545. 10.1016/0960-1686(91)90050-H. [DOI] [Google Scholar]
- Mucha A. P.; Almeida C. M. R.; Bordalo A. A.; Vasconcelos M. T. S. D. Exudation of organic acids by a marsh plant and implications on trace metal availability in the rhizosphere of estuarine sediments. Estuarine, Coastal Shelf Sci. 2005, 65 (1), 191–198. 10.1016/j.ecss.2005.06.007. [DOI] [Google Scholar]
- Keene W. C.; Galloway J. N. Considerations regarding sources for formic and acetic acids in the troposphere. J. Geophys. Res.: Atmos. 1986, 91 (D13), 14466–14474. 10.1029/JD091iD13p14466. [DOI] [Google Scholar]
- Xia L.; Gao Y. Chemical composition and size distributions of coastal aerosols observed on the US East Coast. Mar. Chem. 2010, 119 (1), 77–90. 10.1016/j.marchem.2010.01.002. [DOI] [Google Scholar]
- Zou J.; Liu Z.; Hu B.; Huang X.; Wen T.; Ji D.; Liu J.; Yang Y.; Yao Q.; Wang Y. Aerosol chemical compositions in the North China Plain and the impact on the visibility in Beijing and Tianjin. Atmos. Res. 2018, 201, 235–246. 10.1016/j.atmosres.2017.09.014. [DOI] [Google Scholar]
- Castanho A. D. A.; Artaxo P.; Martins J. V.; Hobbs P. V.; Remer L.; Yamasoe M.; Colarco P. R. Chemical Characterization of Aerosols on the East Coast of the United States Using Aircraft and Ground-Based Stations during the CLAMS Experiment. J. Atmos. Sci. 2005, 62 (4), 934–946. 10.1175/JAS3388.1. [DOI] [Google Scholar]
- Drury E.; Jacob D. J.; Spurr R. J. D.; Wang J.; Shinozuka Y.; Anderson B. E.; Clarke A. D.; Dibb J.; McNaughton C.; Weber R.. Synthesis of satellite (MODIS), aircraft (ICARTT), and surface (IMPROVE, EPA-AQS, AERONET) aerosol observations over eastern North America to improve MODIS aerosol retrievals and constrain surface aerosol concentrations and sources. J. Geophys. Res.: Atmos. 2010, 115 ( (D14), ), 10.1029/2009JD012629. [DOI] [Google Scholar]
- Song C. H.; Carmichael G. R. Gas-Particle Partitioning of Nitric Acid Modulated by Alkaline Aerosol. J. Atmos. Chem. 2001, 40 (1), 1–22. 10.1023/A:1010657929716. [DOI] [Google Scholar]
- Jordan C. E.; Anderson B. E.; Talbot R. W.; Dibb J. E.; Fuelberg H. E.; Hudgins C. H.; Kiley C. M.; Russo R.; Scheuer E.; Seid G.; et al. Chemical and physical properties of bulk aerosols within four sectors observed during TRACE-P. J. Geophys. Res.: Atmos. 2003, 108 ( (D21), ), 10.1029/2002JD003337. [DOI] [Google Scholar]
- Prospero J. M. Long-term measurements of the transport of African mineral dust to the southeastern United States: Implications for regional air quality. J. Geophys. Res.: Atmos. 1999, 104 (D13), 15917–15927. 10.1029/1999JD900072. [DOI] [Google Scholar]
- Zuidema P.; Alvarez C.; Kramer S. J.; Custals L.; Izaguirre M.; Sealy P.; Prospero J. M.; Blades E. Is summer African dust arriving earlier to Barbados? The updated long-term in situ dust mass concentration time series from Ragged Point, Barbados, and Miami, Florida. Bull. Am. Meteorol. Society 2019, 100 (10), 1981–1986. 10.1175/BAMS-D-18-0083.1. [DOI] [Google Scholar]
- Lee Y.-N.; Weber R.; Ma Y.; Orsini D.; Maxwell-Meier K.; Blake D.; Meinardi S.; Sachse G.; Harward C.; Chen T.-Y.; et al. Airborne measurement of inorganic ionic components of fine aerosol particles using the particle-into-liquid sampler coupled to ion chromatography technique during ACE-Asia and TRACE-P. J. Geophys. Res.: Atmos. 2003, 108 ( (D23), ), 10.1029/2002JD003265. [DOI] [Google Scholar]
- Brock C. A.; Sullivan A. P.; Peltier R. E.; Weber R. J.; Wollny A.; de Gouw J. A.; Middlebrook A. M.; Atlas E. L.; Stohl A.; Trainer M. K.; et al. Sources of particulate Emerging investigator series: surfactants..matter in the northeastern United States in summer: 2. Evolution of chemical and microphysical properties. J. Geophys. Res.: Atmos. 2008, 113 ( (D8), ) 10.1029/2007JD009241. [DOI] [Google Scholar]
- Kourtchev I.; O’Connor I. P.; Giorio C.; Fuller S. J.; Kristensen K.; Maenhaut W.; Wenger J. C.; Sodeau J. R.; Glasius M.; Kalberer M. Effects of anthropogenic emissions on the molecular composition of urban organic aerosols: An ultrahigh resolution mass spectrometry study. Atmos. Environ. 2014, 89, 525–532. 10.1016/j.atmosenv.2014.02.051. [DOI] [Google Scholar]
- Burdette T. C.; Frossard A. A. Characterization of seawater and aerosol particle surfactants using solid phase extraction and mass spectrometry. J. Environ. Sci. 2021, 108, 164–174. 10.1016/j.jes.2021.01.026. [DOI] [PubMed] [Google Scholar]
- Cochran R. E.; Laskina O.; Jayarathne T.; Laskin A.; Laskin J.; Lin P.; Sultana C.; Lee C.; Moore K. A.; Cappa C. D.; et al. Analysis of organic anionic surfactants in fine and coarse fractions of freshly emitted sea spray aerosol. Environ. Sci. Technol. 2016, 50 (5), 2477–2486. 10.1021/acs.est.5b04053. [DOI] [PubMed] [Google Scholar]
- Perrie C.; Glenn C. K.; Reed G.; Burdette T. C.; Atwi K.; El Hajj O.; Cheng Z.; Kumar K. V.; Frossard A. A.; Mani S.; Saleh R. Effect of Torrefaction on Aerosol Emissions at Combustion Temperatures Relevant for Domestic Burning and Power Generation. ACS Earth Space Chem. 2022, 6 (11), 2722–2731. 10.1021/acsearthspacechem.2c00251. [DOI] [Google Scholar]
- Tang J.; Li J.; Su T.; Han Y.; Mo Y.; Jiang H.; Cui M.; Jiang B.; Chen Y.; Tang J.; et al. Molecular compositions and optical properties of dissolved brown carbon in biomass burning, coal combustion, and vehicle emission aerosols illuminated by excitation–emission matrix spectroscopy and Fourier transform ion cyclotron resonance mass spectrometry analysis. Atmos. Chem. Phys. 2020, 20 (4), 2513–2532. 10.5194/acp-20-2513-2020. [DOI] [Google Scholar]
- Wozniak A. S.; Bauer J. E.; Sleighter R. L.; Dickhut R. M.; Hatcher P. G. Technical Note: Molecular characterization of aerosol-derived water soluble organic carbon using ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Atmos. Chem. Phys. 2008, 8 (17), 5099–5111. 10.5194/acp-8-5099-2008. [DOI] [Google Scholar]
- Wokosin K. A.; Schell E. L.; Faust J. A. Emerging investigator series: Surfactants, Films, and Coatings on Atmospheric Aerosol Particles: A Review. Environ. Sci.: Atmos. 2022, 2, 775. 10.1039/D2EA00003B. [DOI] [Google Scholar]
- Tervahattu H.; Juhanoja J.; Vaida V.; Tuck A. F.; Niemi J. V.; Kupiainen K.; Kulmala M.; Vehkamäki H.. Fatty acids on continental sulfate aerosol particles. J. Geophys. Res.: Atmos. 2005, 110 ( (D6), ), 10.1029/2004JD005400. [DOI] [Google Scholar]
- Kronberg B.; Holmberg K.; Lindman B.. Surface Chemistry of Surfactants and Polymers; Wiley, 2014, 10.1002/9781118695968. [DOI] [Google Scholar]
- Modini R. L.; Russell L. M.; Deane G. B.; Stokes M. D. Effect of soluble surfactant on bubble persistence and bubble-produced aerosol particles. J. Geophys. Res.-Atmos. 2013, 118 (3), 1388–1400. 10.1002/jgrd.50186. [DOI] [Google Scholar]
- Frossard A. A.; Gérard V.; Duplessis P.; Kinsey J. D.; Lu X.; Zhu Y.; Bisgrove J.; Maben J. R.; Long M. S.; Chang R.; et al. Properties of seawater surfactants associated with primary marine aerosol particles produced by bursting bubbles at a model air-sea interface. Environ. Sci. Technol. 2019, 53, 9407–9417. 10.1021/acs.est.9b02637. [DOI] [PubMed] [Google Scholar]
- Shaharom S.; Latif M. T.; Khan M. F.; Yusof S. N. M.; Sulong N. A.; Abd Wahid N. B.; Uning R.; Suratman S. Surfactants in the sea surface microlayer, subsurface water and fine marine aerosols in different background coastal areas. Environ. Sci. Pollut. Res. 2018, 25 (27), 27074–27089. 10.1007/s11356-018-2745-0. [DOI] [PubMed] [Google Scholar]
- Ćosović B.; Vojvodić V. Voltammetric analysis of surface active substances in natural seawater. Electroanalysis 1998, 10 (6), 429–434. . [DOI] [Google Scholar]
- Gašparović B.; Ćosović B. Electrochemical estimation of the dominant type of surface active substances in seawater samples using o-nitrophenol as a probe. Mar. Chem. 1994, 46 (1–2), 179–188. 10.1016/0304-4203(94)90054-X. [DOI] [Google Scholar]
- Jaafar S. A.; Latif M. T.; Razak I. S.; Abd Wahid N. B.; Khan M. F.; Srithawirat T. Composition of carbohydrates, surfactants, major elements and anions in PM2.5 during the 2013 Southeast Asia high pollution episode in Malaysia. Particuology 2018, 37, 119–126. 10.1016/j.partic.2017.04.012. [DOI] [Google Scholar]
- Roslan R. N.; Hanif N. M.; Othman M. R.; Azmi W.; Yan X. X.; Ali M. M.; Mohamed C. A. R.; Latif M. T. Surfactants in the sea-surface microlayer and their contribution to atmospheric aerosols around coastal areas of the Malaysian peninsula. Mar. Pollut. Bull. 2010, 60 (9), 1584–1590. 10.1016/j.marpolbul.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Latif M. T.; Brimblecombe P. Surfactants in Atmospheric Aerosols. Environ. Sci. Technol. 2004, 38 (24), 6501–6506. 10.1021/es049109n. [DOI] [PubMed] [Google Scholar]
- Baduel C.; Noziere B.; Jaffrezo J. L. Summer/winter variability of the surfactants in aerosols from Grenoble, France. Atmos. Environ. 2012, 47, 413–420. 10.1016/j.atmosenv.2011.10.040. [DOI] [Google Scholar]
- Gérard V.; Noziere B.; Fine L.; Ferronato C.; Singh D. K.; Frossard A. A.; Cohen R. C.; Asmi E.; Lihavainen H.; Kivekäs N.; et al. Concentrations and Adsorption Isotherms for Amphiphilic Surfactants in PM1 Aerosols from Different Regions of Europe. Environ. Sci. Technol. 2019, 53 (21), 12379–12388. 10.1021/acs.est.9b03386. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y.; Gowda D.; Tachibana E.; Takahashi Y.; Hiura T. Identification of secondary fatty alcohols in atmospheric aerosols in temperate forests. Biogeosciences 2019, 16 (10), 2181–2188. 10.5194/bg-16-2181-2019. [DOI] [Google Scholar]
- Bramblett R. L.; Frossard A. A. Constraining the Effect of Surfactants on the Hygroscopic Growth of Model Sea Spray Aerosol Particles. J. Phys. Chem. A 2022, 126 (46), 8695–8710. 10.1021/acs.jpca.2c04539. [DOI] [PubMed] [Google Scholar]
- Frossard A. A.; Li W.; Gerard V.; Noziere B.; Cohen R. C. Influence of surfactants on growth of individual aqueous coarse mode aerosol particles. Aerosol Sci. Technol. 2018, 52 (4), 459–469. 10.1080/02786826.2018.1424315. [DOI] [Google Scholar]
- Petters S. S.; Petters M. D. Surfactant effect on cloud condensation nuclei for two-component internally mixed aerosols. J. Geophys. Res.-Atmos. 2016, 121 (4), 1878–1895. 10.1002/2015JD024090. [DOI] [Google Scholar]
- Ruehl C. R.; Davies J. F.; Wilson K. R. An interfacial mechanism for cloud droplet formation on organic aerosols. Science 2016, 351 (6280), 1447–1450. 10.1126/science.aad4889. [DOI] [PubMed] [Google Scholar]
- Ovadnevaite J.; Zuend A.; Laaksonen A.; Sanchez K. J.; Roberts G.; Ceburnis D.; Decesari S.; Rinaldi M.; Hodas N.; Facchini M. C.; et al. Surface tension prevails over solute effect in organic-influenced cloud droplet activation. Nature 2017, 546 (7660), 637–641. 10.1038/nature22806. [DOI] [PubMed] [Google Scholar]
- Burdette T. C.; Bramblett R. L.; Deegan A. M.; Coffey N. R.; Wozniak A. S.; Frossard A. A. Organic Signatures of Surfactants and Organic Molecules in Surface Microlayer and Subsurface Water of Delaware Bay. ACS Earth Space Chem. 2022, 6 (12), 2929–2943. 10.1021/acsearthspacechem.2c00220. [DOI] [Google Scholar]
- Marple V. A.; Rubow K. L.; Behm S. M. A microorifice uniform deposit impactor (moudi) - description, calibration, and use. Aerosol Sci. Technol. 1991, 14 (4), 434–446. 10.1080/02786829108959504. [DOI] [Google Scholar]
- Frossard A. A.; Long M. S.; Keene W. C.; Duplessis P.; Kinsey J. D.; Maben J. R.; Kieber D. J.; Chang R. Y.-W.; Beaupré S. R.; Cohen R. C.; et al. Marine aerosol production via detrainment of bubble plumes generated in natural seawater with a forced-air venturi. J. Geophys. Res. - Atmos. 2019, 124, 10931. 10.1029/2019JD030299. [DOI] [Google Scholar]
- Quinn P. K.; Coffman D. J. Local closure during the First Aerosol Characterization Experiment (ACE 1): Aerosol mass concentration and scattering and backscattering coefficients. J. Geophys. Res.: Atmos. 1998, 103 (D13), 16575–16596. 10.1029/97JD03757. [DOI] [Google Scholar]
- Khlystov A.; Stanier C.; Pandis S. An algorithm for combining electrical mobility and aerodynamic size distributions data when measuring ambient aerosol special issue of aerosol science and technology on findings from the fine particulate matter supersites program. Aerosol Sci. Technol. 2004, 38 (S1), 229–238. 10.1080/02786820390229543. [DOI] [Google Scholar]
- Freitag S.; Clarke A.; Howell S.; Kapustin V.; Campos T.; Brekhovskikh V.; Zhou J. Combining airborne gas and aerosol measurements with HYSPLIT: a visualization tool for simultaneous evaluation of air mass history and back trajectory consistency. Atmos. Measur. Techn. 2014, 7 (1), 107. 10.5194/amt-7-107-2014. [DOI] [Google Scholar]
- Keene W. C.; Long M. S.; Reid J. S.; Frossard A. A.; Kieber D. J.; Maben J. R.; Russell L. M.; Kinsey J. D.; Quinn P. K.; Bates T. S. Factors that modulate properties of primary marine aerosol generated from ambient seawater on ships at sea. J. Geophys. Res.-Atmos. 2017, 122 (21), 11961–11990. 10.1002/2017JD026872. [DOI] [Google Scholar]
- Noziere B.; Gerard V.; Baduel C.; Ferronato C. Extraction and Characterization of Surfactants from Atmospheric Aerosols. JoVE 2017, 122, 8. 10.3791/55622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard V.; Nozière B.; Baduel C.; Fine L.; Frossard A. A.; Cohen R. C. Anionic, cationic, and nonionic surfactants in atmospheric aerosols from the Baltic coast at Asko, Sweden: Implications for cloud droplet activation. Environ. Sci. Technol. 2016, 50 (6), 2974–2982. 10.1021/acs.est.5b05809. [DOI] [PubMed] [Google Scholar]
- Stubbins A.; Spencer R. G. M.; Chen H.; Hatcher P. G.; Mopper K.; Hernes P. J.; Mwamba V. L.; Mangangu A. M.; Wabakanghanzi J. N.; Six J. Illuminated darkness: Molecular signatures of Congo River dissolved organic matter and its photochemical alteration as revealed by ultrahigh precision mass spectrometry. Limnol. Oceanogr. 2010, 55 (4), 1467–1477. 10.4319/lo.2010.55.4.1467. [DOI] [Google Scholar]
- Kendrick E. A Mass Scale Based on CH2 = 14.0000 for High Resolution Mass Spectrometry of Organic Compounds. Anal. Chem. 1963, 35 (13), 2146–2154. 10.1021/ac60206a048. [DOI] [Google Scholar]
- Hughey C. A.; Hendrickson C. L.; Rodgers R. P.; Marshall A. G.; Qian K. Kendrick Mass Defect Spectrum: A Compact Visual Analysis for Ultrahigh-Resolution Broadband Mass Spectra. Anal. Chem. 2001, 73 (19), 4676–4681. 10.1021/ac010560w. [DOI] [PubMed] [Google Scholar]
- Brechtel F. J.; Kreidenweis S. M.; Swan H. B. Air mass characteristics, aerosol particle number concentrations, and number size distributions at Macquarie Island during the First Aerosol Characterization Experiment (ACE 1). J. Geophys. Res.: Atmos. 1998, 103 (D13), 16351–16367. 10.1029/97JD03014. [DOI] [Google Scholar]
- Bates T. S.; Quinn P. K.; Covert D. S.; Coffman D. J.; Johnson J. E.; Wiedensohler A. Aerosol physical properties and processes in the lower marine boundary layer: a comparison of shipboard sub-micron data from ACE-1 and ACE-2. Tellus B 2000, 52 (2), 258–272. 10.1034/j.1600-0889.2000.00021.x. [DOI] [Google Scholar]
- Lin P.; Hu M.; Wu Z.; Niu Y.; Zhu T. Marine aerosol size distributions in the springtime over China adjacent seas. Atmos. Environ. 2007, 41 (32), 6784–6796. 10.1016/j.atmosenv.2007.04.045. [DOI] [Google Scholar]
- Hoppel W. A.; Fitzgerald J. W.; Larson R. E. Aerosol size distributions in air masses advecting off the east coast of the United States. J. Geophys. Res.: Atmos. 1985, 90 (D1), 2365–2379. 10.1029/JD090iD01p02365. [DOI] [Google Scholar]
- Prospero J. M.; Mayol-Bracero O. L. Understanding the transport and impact of African dust on the Caribbean Basin. Bull. Am. Meteorol. Soc. 2013, 94 (9), 1329–1337. 10.1175/BAMS-D-12-00142.1. [DOI] [Google Scholar]
- Zhao Y.; Gao Y. Mass size distributions of water-soluble inorganic and organic ions in size-segregated aerosols over metropolitan Newark in the US east coast. Atmos. Environ. 2008, 42 (18), 4063–4078. 10.1016/j.atmosenv.2008.01.032. [DOI] [Google Scholar]
- Malm W. C.; Sisler J. F. Spatial patterns of major aerosol species and selected heavy metals in the United States. Fuel Process. Technol. 2000, 65–66, 473–501. 10.1016/S0378-3820(99)00111-3. [DOI] [Google Scholar]
- Harrison R. M.; Pio C. A. Size-differentiated composition of inorganic atmospheric aerosols of both marine and polluted continental origin. Atmos. Environ. (1967) 1983, 17 (9), 1733–1738. 10.1016/0004-6981(83)90180-4. [DOI] [Google Scholar]
- Lewis E. R.; Schwartz S. E.. Sea Salt Aerosol Production: Mechanisms, Methods, Measurements, and Models—A Critical Review; American Geophysical Union, 2004. [Google Scholar]
- Corral A. F.; Dadashazar H.; Stahl C.; Edwards E.-L.; Zuidema P.; Sorooshian A. Source Apportionment of Aerosol at a Coastal Site and Relationships with Precipitation Chemistry: A Case Study over the Southeast United States. Atmosphere 2020, 11 (11), 1212. 10.3390/atmos11111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauscher M. D.; Wang Y.; Moore M. J.; Gaston C. J.; Prather K. A. Air quality impact and physicochemical aging of biomass burning aerosols during the 2007 San Diego wildfires. Environ. Sci. Technol. 2013, 47 (14), 7633–7643. 10.1021/es4004137. [DOI] [PubMed] [Google Scholar]
- Li J.; Pósfai M.; Hobbs P. V.; Buseck P. R.. Individual aerosol particles from biomass burning in southern Africa: 2, Compositions and aging of inorganic particles. J. Geophys. Res.: Atmos. 2003, 108 ( (D13), ), 10.1029/2002JD002310 [DOI] [Google Scholar]
- Axson J. L.; May N. W.; Colón-Bernal I. D.; Pratt K. A.; Ault A. P. Lake Spray Aerosol: A Chemical Signature from Individual Ambient Particles. Environ. Sci. Technol. 2016, 50 (18), 9835–9845. 10.1021/acs.est.6b01661. [DOI] [PubMed] [Google Scholar]
- Frey R. W.; Pinet P. R. Calcium-carbonate content of surficial sands seaward of Altamaha and Doboy Sounds, Georgia. J. Sediment. Res. 1978, 48 (4), 1249–1256. 10.1306/212F764E-2B24-11D7-8648000102C1865D. [DOI] [Google Scholar]
- Herring J. R.; Manheim F. T.; Farrell K. M.; Huddlestun P. F.; Bretz B.. Size Analysis, Visual Estimation of Phosphate and Other Minerals, and Preliminary Estimation of Recoverable Phosphate in Size Fractions of Sediment Samples from Drillholes GAT-90, Tybee Island, and GAS-90-2, Skidaway Island, Georgia; Citeseer, 1991. [Google Scholar]
- Cai W. J.; Wang Y. The chemistry, fluxes, and sources of carbon dioxide in the estuarine waters of the Satilla and Altamaha Rivers Georgia. Limnol. Oceanogr. 1998, 43 (4), 657–668. 10.4319/lo.1998.43.4.0657. [DOI] [Google Scholar]
- Shen Z.; Wang X.; Zhang R.; Ho K.; Cao J.; Zhang M. Chemical composition of water-soluble ions and carbonate estimation in spring aerosol at a semi-arid site of Tongyu. China Aerosol Air Quality Res. 2011, 11 (4), 360–368. 10.4209/aaqr.2011.02.0010. [DOI] [Google Scholar]
- Weinzierl B.; Petzold A.; Esselborn M.; Wirth M.; Rasp K.; Kandler K.; SchüTz L.; Koepke P.; Fiebig M. Airborne measurements of dust layer properties, particle size distribution and mixing state of Saharan dust during SAMUM 2006. Tellus B: Chem. Phys. Meteorol. 2022, 61 (1), 96–117. 10.1111/j.1600-0889.2008.00392.x. [DOI] [Google Scholar]
- Keene W. C.; Sander R.; Pszenny A. A. P.; Vogt R.; Crutzen P. J.; Galloway J. N. Aerosol pH in the marine boundary layer: A review and model evaluation. J. Aerosol Sci. 1998, 29 (3), 339–356. 10.1016/S0021-8502(97)10011-8. [DOI] [Google Scholar]
- Buseck P. R.; Pósfai M. Airborne minerals and related aerosol particles: Effects on climate and the environment. Proc. Natl. Acad. Sci. U. S. A. 1999, 96 (7), 3372–3379. 10.1073/pnas.96.7.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L.; Zhang Y.-Y.; Xiao H.-Y.; Xiao H.-W.; Zheng N.-J.; Zhang Z.-Y.; Xie Y.-J.; Liu C. Spatial distributions and sources of inorganic chlorine in PM2. 5 across China in winter. Atmosphere 2019, 10 (9), 505. 10.3390/atmos10090505. [DOI] [Google Scholar]
- Meng Z.; Seinfeld J. H.; Saxena P.; Kim Y. P. Atmospheric Gas-Aerosol Equilibrium: IV. Thermodynamics of Carbonates. Aerosol Sci. Technol. 1995, 23 (2), 131–154. 10.1080/02786829508965300. [DOI] [Google Scholar]
- Gillette D. A.; Stensland G. J.; Williams A. L.; Barnard W.; Gatz D.; Sinclair P. C.; Johnson T. C. Emissions of alkaline elements calcium, magnesium, potassium, and sodium from open sources in the contiguous United States. Global Biogeochem. Cycles 1992, 6 (4), 437–457. 10.1029/91GB02965. [DOI] [Google Scholar]
- Yang P.; Yang H.; Wang N.; Du C.; Pang S.; Zhang Y. Hygroscopicity measurement of sodium carbonate, β-alanine and internally mixed β-alanine/Na2CO3 particles by ATR-FTIR. J. Environ. Sci. 2020, 87, 250–259. 10.1016/j.jes.2019.07.002. [DOI] [PubMed] [Google Scholar]
- Talbot R. W.; Beecher K. M.; Harriss R. C.; Cofer W. R. III Atmospheric geochemistry of formic and acetic acids at a mid-latitude temperate site. J. Geophys. Res.: Atmos. 1988, 93 (D2), 1638–1652. 10.1029/JD093iD02p01638. [DOI] [Google Scholar]
- Tsai Y. I.; Sopajaree K.; Chotruksa A.; Wu H.-C.; Kuo S.-C. Source indicators of biomass burning associated with inorganic salts and carboxylates in dry season ambient aerosol in Chiang Mai Basin. Thailand. Atmos. Environ. 2013, 78, 93–104. 10.1016/j.atmosenv.2012.09.040. [DOI] [Google Scholar]
- Rivas-Ubach A.; Liu Y.; Bianchi T. S.; Tolić N.; Jansson C.; Paša-Tolić L. Moving beyond the van Krevelen Diagram: A New Stoichiometric Approach for Compound Classification in Organisms. Anal. Chem. 2018, 90 (10), 6152–6160. 10.1021/acs.analchem.8b00529. [DOI] [PubMed] [Google Scholar]
- Reinhardt A.; Emmenegger C.; Gerrits B.; Panse C.; Dommen J.; Baltensperger U.; Zenobi R.; Kalberer M. Ultrahigh Mass Resolution and Accurate Mass Measurements as a Tool To Characterize Oligomers in Secondary Organic Aerosols. Anal. Chem. 2007, 79 (11), 4074–4082. 10.1021/ac062425v. [DOI] [PubMed] [Google Scholar]
- Kleindienst T. E.; Jaoui M.; Lewandowski M.; Offenberg J. H.; Lewis C. W.; Bhave P. V.; Edney E. O. Estimates of the contributions of biogenic and anthropogenic hydrocarbons to secondary organic aerosol at a southeastern US location. Atmos. Environ. 2007, 41 (37), 8288–8300. 10.1016/j.atmosenv.2007.06.045. [DOI] [Google Scholar]
- Lewandowski M.; Piletic I. R.; Kleindienst T. E.; Offenberg J. H.; Beaver M. R.; Jaoui M.; Docherty K. S.; Edney E. O. Secondary organic aerosol characterisation at field sites across the United States during the spring–summer period. Int. J. Environ. Anal. Chem. 2013, 93 (10), 1084–1103. 10.1080/03067319.2013.803545. [DOI] [Google Scholar]
- Koch B. P.; Dittmar T. From mass to structure: an aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun. Mass Spectrom. 2006, 20 (5), 926–932. 10.1002/rcm.2386. [DOI] [Google Scholar]
- Koch B. P.; Dittmar T. From mass to structure: an aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun. Mass Spectrom. 2016, 30 (1), 250–250. 10.1002/rcm.7433. [DOI] [Google Scholar]
- Oppo C.; Bellandi S.; Degli Innocenti N.; Stortini A.; Loglio G.; Schiavuta E.; Cini R. Surfactant components of marine organic matter as agents for biogeochemical fractionation and pollutant transport via marine aerosols. Mar. Chem. 1999, 63 (3–4), 235–253. 10.1016/S0304-4203(98)00065-6. [DOI] [Google Scholar]
- Cavalli F.; Facchini M. C.; Decesari S.; Mircea M.; Emblico L.; Fuzzi S.; Ceburnis D.; Yoon Y. J.; O’Dowd C. D.; Putaud J. P.; et al. Advances in characterization of size-resolved organic matter in marine aerosol over the North Atlantic. J. Geophys. Res.-Atmos. 2004, 109 (D24), D24215. 10.1029/2004JD005137. [DOI] [Google Scholar]
- Ohlrogge J.; Thrower N.; Mhaske V.; Stymne S.; Baxter M.; Yang W.; Liu J.; Shaw K.; Shorrosh B.; Zhang M.; et al. PlantFAdb: a resource for exploring hundreds of plant fatty acid structures synthesized by thousands of plants and their phylogenetic relationships. Plant J. 2018, 96 (6), 1299–1308. 10.1111/tpj.14102. [DOI] [PubMed] [Google Scholar]
- Ballentine D. C.; Macko S. A.; Turekian V. C. Variability of stable carbon isotopic compositions in individual fatty acids from combustion of C4 and C3 plants: implications for biomass burning. Chem. Geol. 1998, 152 (1), 151–161. 10.1016/S0009-2541(98)00103-X. [DOI] [Google Scholar]
- Simoneit B. R. T.; Mazurek M. A. Organic matter of the troposphere—II. Natural background of biogenic lipid matter in aerosols over the rural western United States. Atmos. Environ. (1967) 1982, 16 (9), 2139–2159. 10.1016/0004-6981(82)90284-0. [DOI] [Google Scholar]
- Fang J.; Kawamura K.; Ishimura Y.; Matsumoto K. Carbon Isotopic Composition of Fatty Acids in the Marine Aerosols from the Western North Pacific: Implication for the Source and Atmospheric Transport. Environ. Sci. Technol. 2002, 36 (12), 2598–2604. 10.1021/es015863m. [DOI] [PubMed] [Google Scholar]
- Parmentier E. A.; David G.; Arroyo P. C.; Bibawi S.; Esat K.; Signorell R. Photochemistry of single optically trapped oleic acid droplets. J. Aerosol Sci. 2021, 151, 105660 10.1016/j.jaerosci.2020.105660. [DOI] [Google Scholar]
- Kieber R. J.; Hydro L. H.; Seaton P. J. Photooxidation of triglycerides and fatty acids in seawater: Implication toward the formation of marine humic substances. Limnol. Oceanogr. 1997, 42 (6), 1454–1462. 10.4319/lo.1997.42.6.1454. [DOI] [Google Scholar]
- Aiken A. C.; DeCarlo P. F.; Kroll J. H.; Worsnop D. R.; Huffman J. A.; Docherty K. S.; Ulbrich I. M.; Mohr C.; Kimmel J. R.; Sueper D.; et al. O/C and OM/OC Ratios of Primary, Secondary, and Ambient Organic Aerosols with High-Resolution Time-of-Flight Aerosol Mass Spectrometry. Environ. Sci. Technol. 2008, 42 (12), 4478–4485. 10.1021/es703009q. [DOI] [PubMed] [Google Scholar]
- Fu P.; Kawamura K.; Miura K.. Molecular characterization of marine organic aerosols collected during a round-the-world cruise. J. Geophys. Res.: Atmos. 2011, 116 ( (D13), ), 10.1029/2011JD015604. [DOI] [Google Scholar]
- Kundu S.; Kawamura K.; Andreae T.; Hoffer A.; Andreae M. Molecular distributions of dicarboxylic acids, ketocarboxylic acids and α-dicarbonyls in biomass burning aerosols: implications for photochemical production and degradation in smoke layers. Atmos. Chem. Phys. 2010, 10 (5), 2209–2225. 10.5194/acp-10-2209-2010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.