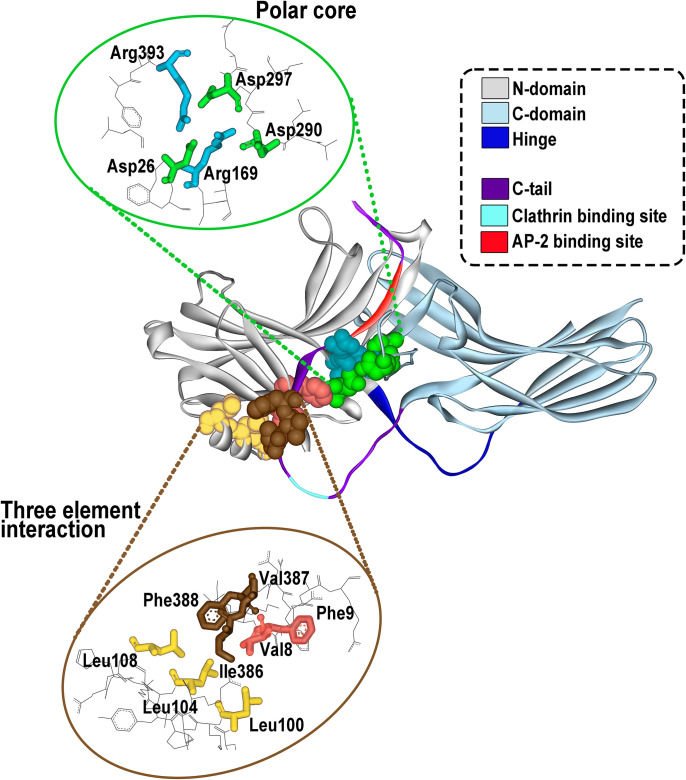
Fig. 1.
Arrestin structure and important functional elements. All arrestins consist of the N-domain (gray), the C-domain (blue-gray), and the C-terminus (C-tail, magenta) that emerges from the C-domain and is anchored to the N-domain via the so-called three-element interaction and the polar core (side chains participating in these two interactions are shown as CPK models on the structure; their spatial arrangement is shown in the insets). The N- and C-domains are connected by a 12-residue hinge region (dark blue). The C-terminus contains binding sites for clathrin (light blue) and clathrin adaptor AP2 (red). The basal arrestin conformation is stabilized by two intramolecular interactions. One is the polar core, an arrangement of five charged side chains, two of which are supplied by the N-domain, two by the C-domain, and one by the C-terminus (upper inset). The other one is the three-element interaction mediated by hydrophobic side chains of β-strand I and α-helix I of the N-domain and β-strand XX of the C-terminus (lower inset). Both of these interactions are destabilized by GPCR binding, which results in the release of the C-terminus and twisting of the two domains relative to each other by approximately 20°.