Abstract
Acetylene gas is an important feedstock for chemical production, although it is underutilized in organic synthesis. We have developed an intermolecular gold(I)-catalyzed alkyne/alkene reaction of o-allylphenols with acetylene gas that gives rise to chromanes by a stereospecific aryloxycyclization through the nucleophilic regioselective opening of cyclopropyl gold(I)-carbene intermediates. The synthetic application of this method was demonstrated in the late-stage functionalization of the natural product lapachol.
Keywords: gold catalysis, acetylene, aryloxycyclization, chromanes, enantioselective catalysis
Acetylene is one of the most important feedstocks in chemical industry due to its ready availability and high reactivity.1,2 Acetylene can be produced by several well-established methods such as the reaction of calcium carbide with water2 or the partial combustion of hydrocarbons.3 The importance of acetylene-based chemistry is best illustrated by its remarkable production market that reached 1.9 million tones in 2020 and is expected to continue growing until 2030.2 Despite this, so far, chemical applications of acetylene have been mainly limited to noncatalyzed vinylation reactions4 or hydrochlorination processes,5 whereas its use in catalytic reactions has been less explored.2,6−8
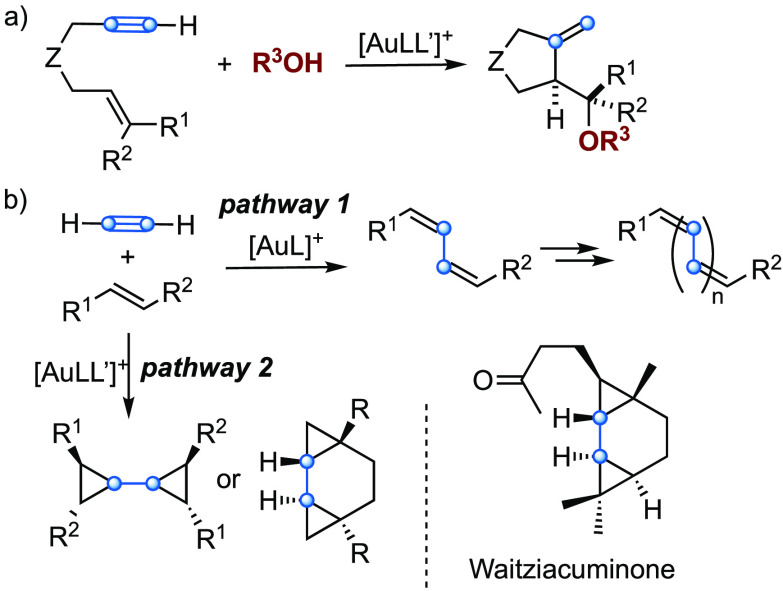
Homogeneous gold(I) complexes are highly efficient catalysts for the electrophilic activation of alkynes.9 Although gold(I)-catalyzed cyclizations of 1,n-enynes,9 such as the alkoxycyclizations by intramolecular alkyne/alkene reactions (Scheme 1a),10 have been widely explored, broad scope intermolecular reactions between alkynes and alkenes are less common.11−13 Besides the possible polymerization of the alkenes,14 the main hurdle is that products of intermolecular reactions of alkynes with alkenes are also alkenes, which can react further with the alkyne leading to the formation of oligomers. Indeed, we recently reported that acetylene gas reacts with trans-stilbene in the presence of gold(I) catalysts to form (Z,Z)-1,3-dienes, along with oligomers that result from the formal insertion of C2 units (Scheme 1b, pathway 1).15 By using a NHC-gold(I) catalyst, biscyclopropyl products were also obtained, which was applied to the first total synthesis of the sesquiterpene waitziacuminone in a single step.15 (Scheme 1b, pathway 2).
Scheme 1. (a) Gold(I)-Catalyzed Alkoxycyclization by Intramolecular Alkyne/Alkene Reaction9,10 and (b) Activation of Acetylene Gas15.
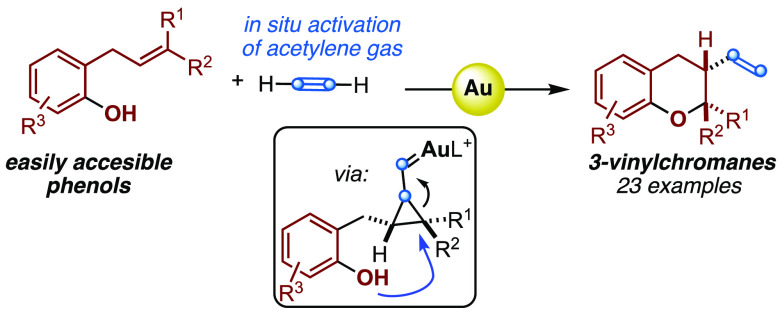
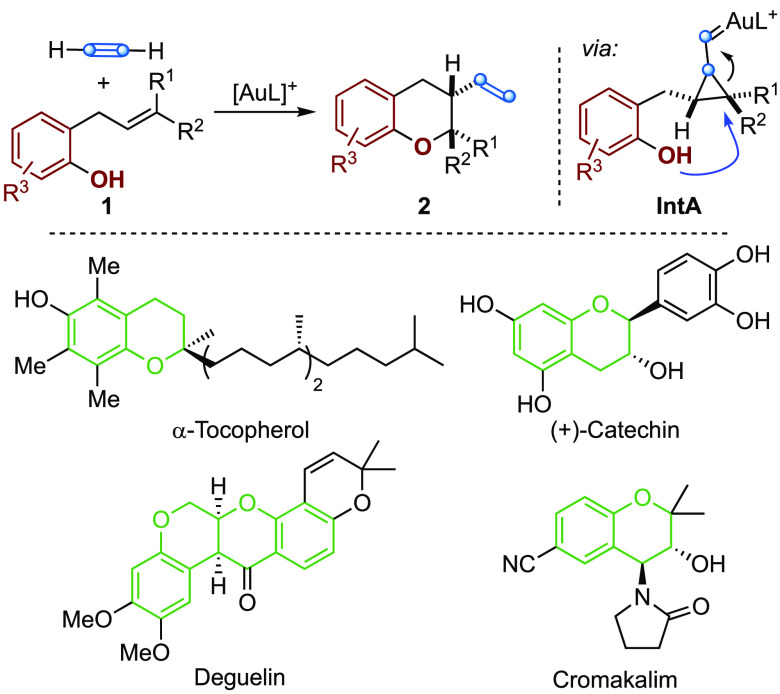
Although alkoxycyclizations of 1,n-enynes are well-known (Scheme 1a),10,16 the intermolecular version has not yet been developed. We reasoned that using acetylene gas as an intermolecular in the gold(I)-catalyzed reaction with an alkene would give rise to products with a terminal vinyl group, which are less reactive in subsequent reactions with acetylene, thus minimizing the problem of oligomerization. Here, we report the realization of this concept by developing an intermolecular alkyne/alkene gold(I)-catalyzed reaction from o-allylphenols 1 and acetylene gas that gives rise stereospecifically to chromanes 2 (Scheme 2). In this aryloxyvinylation reaction, the initial acetylene gold(I) complex is the electrophile that reacts with the alkene to form cyclopropyl gold(I)-carbene IntA, which reacts regioselectively with the phenol at C-3 of the allyl chain to form a 6-membered ring. The resulting chromanes are important heterocyclic scaffolds present in a wide variety of natural products, agrochemical, and pharmaceutical compounds,17 such as α-tocopherol,18 (+)-catechin,19 deguelin,20 and cromakalim.21
Scheme 2. Aryloxyvinylation by Gold(I)-Catalyzed Intermolecular Alkyne/Alkene Reaction.
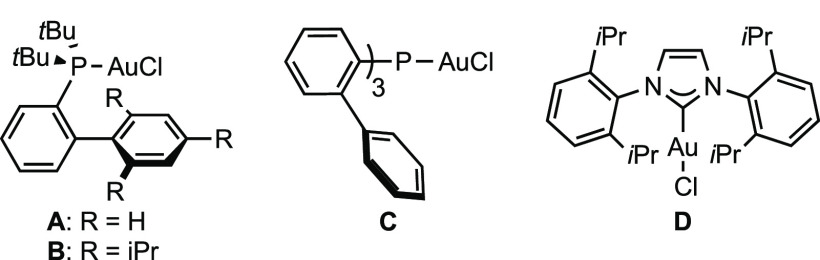
The reaction of 2-cinnamyl phenol (1a) with acetylene gas in the presence of commercially available JohnPhosAuCl (A) as a catalyst and NaBArF4 as a halide scavenger gave the desired vinylated chromane 2a in 78% yield (Table 1, entry 1). Gold(I) catalysts B, C, and D led to 2a in lower yields (Table 1, entries 2–4). Using CHCl3 instead of CH2Cl2 as solvent with catalyst A improved the yield of 2a to 89% (Table 1, entry 5). Aromatic solvents gave comparable yields, toluene being the best one, providing 2a in 81% yield (Table 1, entry 6). Changing NaBArF4 to AgSbF6 as a chloride abstractor led to a drop of the yield (49%) (Table 1, entry 7). Finally, 2a was obtained in 91% yield by increasing the concentration (Table 1, entry 9).22
Table 1. Gold(I)-Catalyzed Reaction of 1a with Acetylene Gas to Form Chromane 2aa.
The optimized reaction conditions were applied to the synthesis of a variety of 3-vinyl chromane derivates 2a–w (Scheme 3). First, the influence of the substituents on the phenol ring was investigated. Substrates with electron-donating groups gave the corresponding products 2b–d, 2h, and 2j–l in moderate to good yields, whereas 2i could only be isolated in 29% yield. An allyl phenol with a Br substituent in the para-position led to chromane 2e in good yield, whereas substitution with more strongly electron-withdrawing ester or CF3 groups led to 2f and 2g in 20% and 33% yield, respectively, presumably because of the decreased nucleophilicity of the corresponding phenols. Substrates with different substituents on the phenyl ring of the cinnamyl chain led to products 2m–t in gold yields, except for 2p and 2r with a p-CF3 or o-Br, which were isolated in 21% and 30% yields, respectively (Scheme 3). Other o-allyl phenols with different substituents at the alkene gave chromanes 2u–w in 37–74% yields.
Scheme 3. Synthesis of Chromanes 2 by Gold(I)-Catalyzed Aryloxyvinylation of o-Allylphenols 1 with Acetylene Gas.
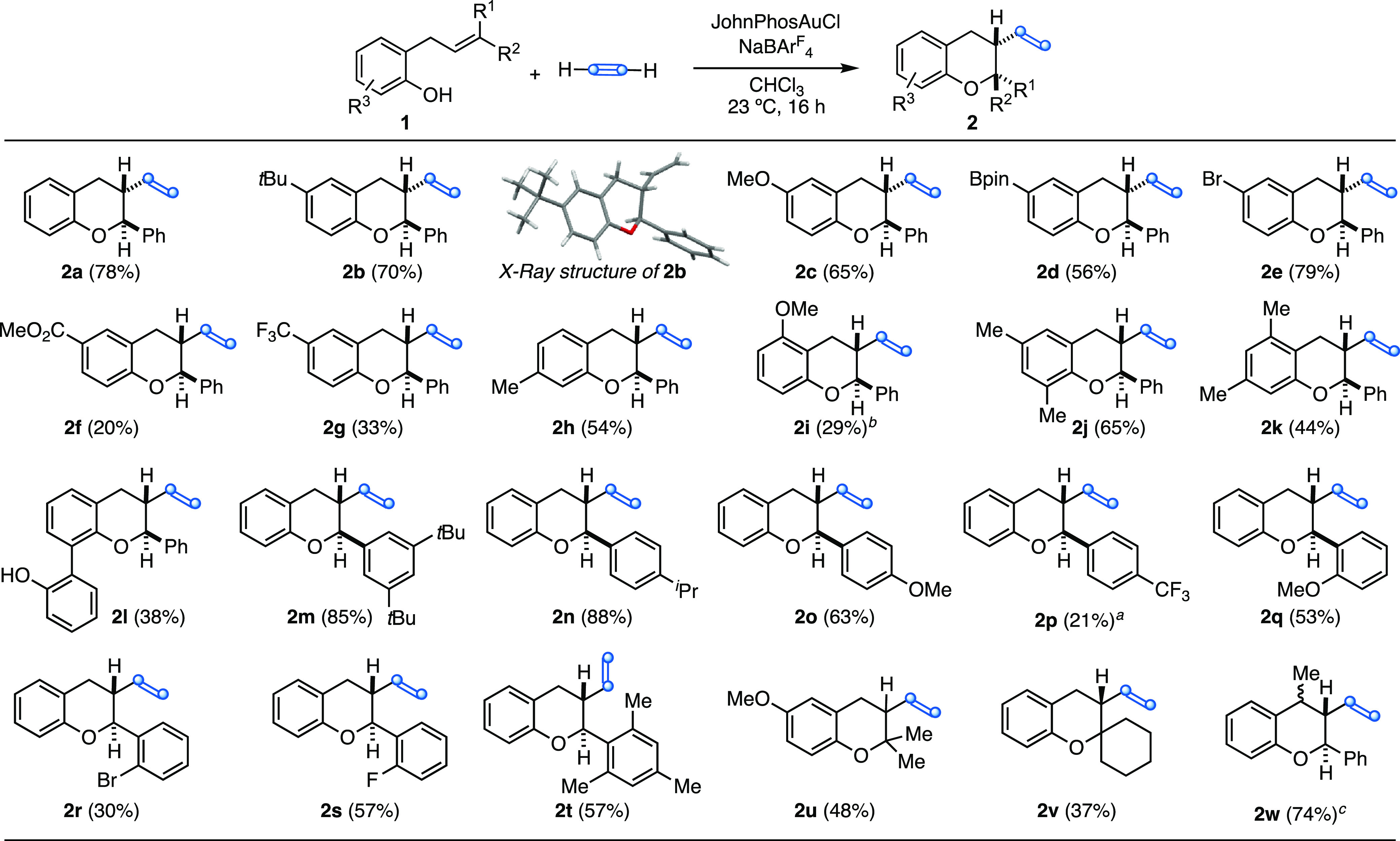
48 h reaction time.
0 °C for 3 h.
1:1 mixture of stereoisomers
The observed anti-stereochemistry and excusive 6-endo-trig regioselectivity is identical to that found in similar formation of chromanes by halocyclization of the same substrates.23 However, in our case, the cyclization is induced by the addition of acetylene as a C2 equiv of the halonium electrophile.
Since o-allylphenols are ubiquitous in nature, this aryloxyvinylation could be used for the late-stage modification of this class of natural products.24 As a preliminary demonstration of this concept, we have applied this new reaction to the natural product lapachol (3), a derivate of vitamin K,25 leading to 3-vinyl-α-lapachone 4 in 50% yield (Scheme 4a). Vinyl chromane 2d was converted into 2x by Suzuki cross-coupling with bromobenzene (Scheme 4b). The vinyl group provides a versatile handle for diversification. Thus, 2a led to 5 by Wacker oxidation, whereas reaction with mCPBA gave 6 (Scheme 4c). Furthermore, metathesis of 2a with methyl acrylate afforded 7, and the hydroboration with HBpin provided 8. A monocationic catalyst generated in situ from JosiPhos-type digold(I) complex (R,SP)-E12d proved to be highly active leading to 2a, 2d, 2e, and 2m (Scheme 4d). Although the achieved enantioselectivities are still moderate, these are the first examples of enantioselective activation of acetylene in gold(I) catalysis.
Scheme 4. (a) Vinylation of Lapachol (3); (b) Derivatization of 2d; (c) Derivatization of 2a; (d) Enantioselective Aryloxycyclization.
In summary, we have developed a gold(I)-catalyzed intermolecular reaction between acetylene gas and readily available o-allylphenols as a novel approach for the synthesis of 3-vinylchromanes. This stereoselective intermolecular aryloxyvinylation leads to chromanes in moderate to excellent yields, showing good functional group tolerance. The applicability of this method was demonstrated by the late-stage functionalization of the natural product lapachol (3) and with the diversification at the aryl or vinyl of the resulting chromanes. This new methodology combines the use of common feedstock reagents such as acetylene gas and phenols with the employment of gold(I) catalysis to obtain scaffolds widely abundant in natural products and pharmacologically active compounds.
Acknowledgments
We thank the MCIN/AEI/10.13039/501100011033 (PID2019-104815GB-I00 and CEX2019-000925-S), the European Research Council (Advanced Grant 835080), the AGAUR (2021 SGR 01256), and CERCA Program/Generalitat de Catalunya for financial support. T.M. thanks the MCIN/AEI for a FPI predoctoral fellowship (PRE2020-092105), A.S. thanks the European Union (H2020 MSCA-COFUND 801474 Postdoctoral Fellowship, GA 801474), L.A.H. thanks the European Union (Horizon 2020 Marie Skłodowska-Curie Postdoctoral Fellowship, H2020-MSCA-IF-2020 under Grant Agreement No. 101029012-GASGOLD). We also thank ICIQ X-ray diffraction, NMR, and mass spectrometry units.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.3c02461.
Experimental procedures, characterization data, NMR data, UPC2 and HPLC traces, computational details, and X-ray data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Trotus I. T.; Zimmermann T.; Schüth F. Catalytic Reactions of Acetylene: A Feedstock for Chemical Industry Revisited. Chem. Rev. 2014, 114, 1761–1782. 10.1021/cr400357r. [DOI] [PubMed] [Google Scholar]
- a Voronin V. V.; Ledovskaya M. S.; Bogachenkov A. S.; Rodygin K. S.; Ananikov V. P. Acetylene in Organic Synthesis: Recent Progress and New Uses. Molecules 2018, 23, 2442–2526. 10.3390/molecules23102442. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rodygin K.; Ledovskaya M.; Voronin V.; Lotsman K.; Ananikov V. P. Calcium Carbide: Versatile Synthetic Applications, Green Methodology and Sustainability. Eur. J. Org. Chem. 2021, 2021, 43–52. 10.1002/ejoc.202001098. [DOI] [Google Scholar]; c Ledovskaya M. S.; Voronin V. V.; Rodygin K. S.; Ananikov V. P. Acetylene and Ethylene: Universal C2Molecular Units in Cycloaddition Reactions. Synthesis 2022, 54, 999–1042. 10.1055/a-1654-2318. [DOI] [Google Scholar]
- Pässler P.; Hefner W.; Buckl K.; Meinass H.; Meiswinkel A.; Wernicke H. J.; Ebersberg G.; Müller R.; Bässler J.; Behringer H.; Mayer D.. Acetylene. Ullmann's Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; p 1. [Google Scholar]
- Ledovskaya M. S.; Voronin V. V.; Rodygin K. S. Methods for the Synthesis of O-, S- and N-Vinyl Derivatives. Russ. Chem. Rev. 2018, 87, 167–191. 10.1070/RCR4782. [DOI] [Google Scholar]
- Tedeschi R. J.Acetylene-Based Chemical from Coal and Other Natural Resources; Dekker: New York, 1982; p 232. [Google Scholar]
- a Yang Bo.; Lu S.; Wang Y.; Zhu S. Diverse Synthesis of C2-linked Functionalized Molecules via Molecular Glue Strategy with Acetylene. Nat. Commun. 2022, 13, 1858–1870. 10.1038/s41467-022-29556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lin Z.; Liu B.; Wang Y.; Li S.; Zhu S. Synthesis of Vinyl-Substituted Alcohols Using Acetylene as C2 Building Block. Chem. Sci. 2023, 14, 1912–1918. 10.1039/D2SC06400F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabugin I. V.; Gold B. Two Functional Groups in One Package: Using both Alkyne π-bonds in Cascade Transformations. J. Org. Chem. 2013, 78, 7777–7784. 10.1021/jo401091w. [DOI] [PubMed] [Google Scholar]
- U.S. Chemical Safety and Hazard Investigation Board (CSB) . Safety Bulletin No. 2005-03-B, January 2006.
- a Dorel R.; Echavarren A. M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. 10.1021/cr500691k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hashmi S. K. Gold-Catalyzed Organic Reactions. Chem. Rev. 2007, 107, 3180–3211. 10.1021/cr000436x. [DOI] [PubMed] [Google Scholar]; c Nieto-Oberhuber C.; López S.; Echavarren A. M. Intramolecular [4 + 2] Cycloadditions of 1,3-Enynes or Arylalkynes with Alkenes with Highly Reactive Cationic Phosphine Au(I) Complexes. J. Am. Chem. Soc. 2005, 127, 6178–6179. 10.1021/ja042257t. [DOI] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; Muñoz M. P.; López S.; Jiménez-Núñez E.; Nevado C.; Herrero-Gómez E.; Raducan M.; Echavarren A. M. Gold(I)-Catalyzed Cyclizations of 1,6-Enynes: Alkoxycyclizations and Exo/Endo Skeletal Rearrangements. Chem.—Eur. J. 2006, 12, 1677–1693. 10.1002/chem.200501088. [DOI] [PubMed] [Google Scholar]
- a Muratore M. E.; Homs A.; Obradors C.; Echavarren A. M. Meeting the Challenge of Intermolecular Gold(I)-Catalyzed Cycloadditions of Alkynes and Allenes. Chem. Asian. J. 2014, 9, 3066–3082. 10.1002/asia.201402395. [DOI] [PMC free article] [PubMed] [Google Scholar]; b García-Morales C.; Echavarren A. M. From Straightforward Gold(I)-Catalyzed Enyne Cyclizations to More Demanding Intermolecular Reactions of Alkynes with Alkenes. Synlett 2018, 29, 2225–2237. 10.1055/s-0037-1610203. [DOI] [Google Scholar]
- a López-Carrillo V.; Echavarren A. M. Gold(I)-Catalyzed Intermolecular [2 + 2] Cycloaddition of Alkynes with Alkenes. J. Am. Chem. Soc. 2010, 132, 9292–9294. 10.1021/ja104177w. [DOI] [PubMed] [Google Scholar]; b Obradors C.; Echavarren A. M. Intermolecular Gold-Catalyzed Cycloaddition of Alkynes with Oxoalkenes. Chem.—Eur. J. 2013, 19, 3547–3551. 10.1002/chem.201300131. [DOI] [PubMed] [Google Scholar]; c Huguet N.; Leboeuf D.; Echavarren A. M. Intermolecular Gold(I)-Catalyzed Cyclization of Furans with Alkynes: Formation of Phenols and Indene. Chem.—Eur. J. 2013, 19, 6581–6585. 10.1002/chem.201300646. [DOI] [PubMed] [Google Scholar]; d García-Morales C.; Ranieri B.; Escofet I.; López-Suarez L.; Obradors C.; Konovalov A. I.; Echavarren A. M. Enantioselective Synthesis of Cyclobutenes by Intermolecular [2 + 2] Cycloaddition with Non-C2 Symmetric Digold Catalysts. J. Am. Chem. Soc. 2017, 139, 13628–13631. 10.1021/jacs.7b07651. [DOI] [PMC free article] [PubMed] [Google Scholar]; e de Orbe M.; Echavarren A. M. Broadening the Scope of the Gold Catalyzed [2 + 2] Cycloaddition: Synthesis of Vinylcyclobutenes and Further Transformations. Eur. J. Org. Chem. 2018, 2018, 2740–2752. 10.1002/ejoc.201800170. [DOI] [Google Scholar]; f Zanini M.; Cataffo A.; Echavarren A. M. Synthesis of Cyclobutanones by Gold(I)-Catalyzed [2 + 2] Cycloaddition of Ynol Ethers with Alkenes. Org. Lett. 2021, 23, 8989–8993. 10.1021/acs.orglett.1c03499. [DOI] [PubMed] [Google Scholar]
- a Yeom H.-S.; Koo J.; Park H.-S.; Wang Y.; Liang Y.; Yu Z.-X.; Shin S. Gold-Catalyzed Intermolecular Reactions of Propiolic Acids with Alkenes: [4 + 2] Annulation and Enyne Cross Metathesis. J. Am. Chem. Soc. 2012, 134, 208–211. 10.1021/ja210792e. [DOI] [PubMed] [Google Scholar]; b Park S. R.; Kim C.; Kim D.; Thrimurtulu N.; Yeom H.-S.; Jun J.; Shin S.; Rhee Y. H. Entry to β-Alkoxyacrylates via Gold-Catalyzed Intermolecular Coupling of Alkynoates and Allylic Ethers. Org. Lett. 2013, 15, 1166–1169. 10.1021/ol4001087. [DOI] [PubMed] [Google Scholar]; c Jun J.; Yeom H.-S.; An J.-H.; Shin S. Gold-Catalyzed Intermolecular Coupling of Sulfonylacetylene with Allyl Ethers: [3,3]- and [1,3]-Rearrangements. Beilstein J. Org. Chem. 2013, 9, 1724–1729. 10.3762/bjoc.9.198. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Kim H.; Choi S. Y.; Shin S. Asymmetric Synthesis of Dihydropyranones via Gold(I)-Catalyzed Intermolecular [4 + 2] Annulation of Propiolates and Alkenes. Angew. Chem., Int. Ed. 2018, 57, 13130–13134. 10.1002/anie.201807514. [DOI] [PubMed] [Google Scholar]
- Urbano J.; Hormigo A. J.; de Frémont P.; Nolan P. S.; Díaz-Requejo M. M.; Pérez P. J. Gold-promoted Styrene Polymerization. Chem. Commun. 2008, 759–761. 10.1039/B716145J. [DOI] [PubMed] [Google Scholar]
- Scharnagel D.; Escofet I.; Armengol-Relats H.; de Orbe M. E.; Korber J. N.; Echavarren A. M. Acetylene as a Dicarbene Equivalent for Gold(I) Catalysis: Total Synthesis of Waitziacuminone in One Step. Angew. Chem., Int. Ed. 2020, 59, 4888–4891. 10.1002/anie.201915895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echavarren A. M.; Muratore M. N.; López-Carrillo V.; Escribano-Cuesta A.; Huguet A.; Obradors C. Gold-catalyzed cyclizations of alkynes with alkenes and arenes. Org. React. 2017, 92, 1–288. 10.1002/0471264180.or092.01. [DOI] [Google Scholar]
- Li W.; Shuai W.; Xu F.; Sun H.; Xu S.; Yao H.; Liu J.; Yao H.; Zhu Z.; Xu J. Discovery of Novel 4-Arylisochromenes as Anticancer Agents Inhibiting Tubulin Polymerization. ACS Med. Chem. Lett. 2018, 9, 974–979. 10.1021/acsmedchemlett.8b00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer J. E.; Slater T. F.; Willson R. L. Direct Observation of a Free Radical Interaction between Vitamin E and Vitamin C. Nature 1979, 278, 737–738. 10.1038/278737a0. [DOI] [PubMed] [Google Scholar]
- Raab T.; Barron D.; Vera F. A.; Crespy V.; Oliveira M.; Williamson G. Catechin Glucosides: Occurrence, Synthesis, and Stability. J. Agric. Food Chem. 2010, 58, 2138–2149. 10.1021/jf9034095. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Ma W.; Zheng W. Deguelin, a Novel Anti-tumorigenic Agent Targeting Apoptosis, Cell Cycle Arrest and Anti-angiogenesis for Cancer Chemoprevention. Mol. Clin. Oncol. 2013, 1, 215–219. 10.3892/mco.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. C.; Weston A. H. Cromakalim, Nicorandil and Pinacidil: Novel Drugs which Open Potassium Channels in Smooth muscle. Gen. Pharmac. 1989, 20, 1–9. 10.1016/0306-3623(89)90052-9. [DOI] [PubMed] [Google Scholar]
- See Supporting Information for additional details.
- Lu Y.; Nakatsuji H.; Okumura Y.; Yao L.; Ishihara K. Enantioselective Halo-oxy and Halo-azacyclizations Induced by Chiral Amidophosphate Catalysts and Halo-Lewis Acids. J. Am. Chem. Soc. 2018, 140, 6039–6043. 10.1021/jacs.8b02607. [DOI] [PubMed] [Google Scholar]
- Guillemard L.; Kaplaneris N.; Ackermann L.; Johansson M. G. Late-Stage C–H Functionalization Offers New Oportunities in Drug Discovery. Nat. Rev. Chem. 2021, 5, 522–545. 10.1038/s41570-021-00300-6. [DOI] [PubMed] [Google Scholar]
- Epifano F.; Genovese S.; Fiorito S.; Mathieu V.; Kiss R. Lapachol and its congeners as Anticancer Agents: a Review. Phytochem. Rev. 2014, 13, 37–49. 10.1007/s11101-013-9289-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.