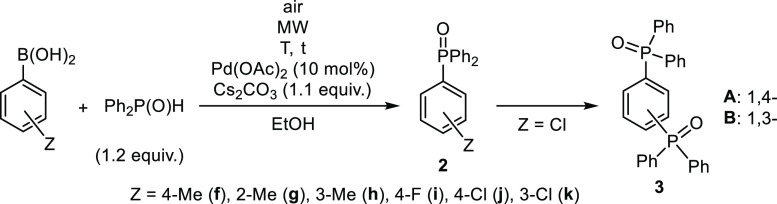
Table 3. P–C Coupling of Substituted Arylboronic Acids with Diphenylphosphine Oxide.
| product
composition (%)a,b |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| entry | Z | T | t (h) | conversion (%)a | 2 | 3 | (EtO)Ph2PO | Ph3PO (1a) | yield (%) |
| 1 | H | 135 | 1.5 | 100b | 95 (1a) | 5 | 83 (1a) | ||
| 2 | 4-Me (f) | 135 | 1.5 | 100 | 81 | 5 | 14 | 73 (2f) | |
| 3 | 2-Me (g) | 135 | 1.5 | 100 | 71 | 13 | 16 | 67 (2g) | |
| 4 | 3-Me (h) | 135 | 1.5 | 100 | 87 | 8 | 5 | 80 (2h) | |
| 5 | 4-F (i) | 135 | 1.5 | 100 | 75 | 10 | 15 | 70 (2i) | |
| 6 | 4-Cl (j) | 135 | 1.5 | 100 | 49 (3A) | 51 | 23 (3A) | ||
| 7 | 4-Cl (j) | 90 | 4 | 100 | 46 | 5 | 11 | 38 | (2j)c |
| 8 | 4-Cl (j) | 90d | 4 | 77 | 12 | 41 (3A) | 6 | 18 | |
| 9 | 4-Cl (j) | 135e | 1 | 100 | 23 (3A) | 5 | 72 | ||
| 10 | 3-Cl (k) | 135 | 1.5 | 100 | 53 (3B) | 6 | 41 | 25 (3B) | |
| 11 | 3-Cl (k) | 90 | 4 | 96 | 35 | 22 (3B) | 11 | 28 | (2k)f |
| 12 | 3-Cl (k) | 90c | 22 | 100 | 33 | 44 (3B) | 4 | 19 | |
On the basis of relative 31P NMR intensities of the P-components.
The average of two or three parallel experiments.
31P NMR (CDCl3) δ 28.5, δP lit.23 (CDCl3) 28.2; HRMS (m/z): calcd for C18H15OPCl [M + H]+, 313.0549; found, 313.0542.
On conventional heating.
2.4 equiv of Ph2P(O)H was used.
31P NMR (CDCl3) δ 28.1, δP lit.24 (CDCl3) 28.1; HRMS (m/z): calcd for C18H15OPCl [M + H]+, 313.0549; found, 313.0538.