Abstract

Among phenol-derived electrophiles, aryl sulfamates are attractive substrates since they can be employed as directing groups for C–H functionalization prior to catalysis. However, their use in C–N coupling is limited only to Ni catalysis. Here, we describe a Pd-based catalyst with a broad scope for the amination of aryl sulfamates. We show that the N-methyl-2-aminobiphenyl palladacycle supported by the PCyp2ArXyl2 ligand (Cyp = cyclopentyl; ArXyl2 = 2,6-bis(2,6-dimethylphenyl)phenyl) efficiently catalyzes the C–N coupling of aryl sulfamates with a variety of nitrogen nucleophiles, including anilines, primary and secondary alkyl amines, heteroaryl amines, N-heterocycles, and primary amides. DFT calculations support that the oxidative addition of the aryl sulfamate is the rate-determining step. The C–N coupling takes place through a cationic pathway in the polar protic medium.
Keywords: amination, palladacycle, phosphine, DFT calculations, microkinetic modeling, aryl sulfamates
Introduction
In recent years, phenol derivatives have gained popularity as viable surrogates for aryl halide electrophiles in transition metal-catalyzed cross-coupling chemistry.1 In particular, nickel catalysts allow for the activation and subsequent functionalization of a variety of phenol-derive electrophiles, including less reactive aryl ethers.2,3 Despite the tremendous advances made with palladium catalysts in terms of versatility, substrate scope, functional group compatibility, and milder reaction conditions, the use of phenol derivatives is basically limited to more reactive aryl sulfonates (e.g., aryl triflates and tosylates) in Pd-catalyzed reactions.4
Unlike sulfonate derivatives, aryl sulfamates are appealing substrates since they can be used as directing groups for C–H functionalization5 and show superior stability within the wide range of experimental conditions applied in cross-coupling reactions. Given the ability of Ni to cleave C(sp2)–O bonds,6 most reported protocols with sulfamates as reaction partners are based on the use of Ni catalysts.7 To date, only a few examples with aryl sulfamates as electrophiles in palladium-catalyzed C–C bond formation have been reported.8 Of these, the room-temperature Suzuki–Miyaura coupling of aryl sulfamates described by Hazari and co-workers is of particular note.8c,8d The authors ascribe the excellent performance of their catalytic system to the extra stabilization provided by the noncovalent interaction between the Pd center and the biaryl motif of the XPhos ligand.8d This secondary interaction is key to lowering the energy of the transition state for oxidative addition, the turnover-limiting step.
Recently, we reported on the synthesis of a family of dialkylterphenyl phosphines and discussed their steric and electronic properties.9 These phosphines can adopt different coordination modes involving the P atom and one of the flanking aryl rings of the terphenyl fragment.9a,10 We showed that 2-aminobiphenyl palladacycles ligated with the bulkier ligand PCyp2ArXyl2 (Cyp = cyclopentyl; ArXyl2 = 2,6-bis(2,6-dimethylphenyl)phenyl) are excellent precatalysts for the Buchwald–Hartwig reaction of deactivated aryl chlorides with a variety of N-nucleophiles.11 To expand the array of electrophiles that can be applied in this transformation, we focused on challenging aryl sulfamates, since, to our knowledge, they have not been used as coupling partners in palladium-catalyzed Buchwald–Hartwig aminations.4,12 Furthermore, the substrate scope of Ni catalysts reported for the amination of aryl sulfamates remains limited to anilines and secondary alkyl amines (Figure 1A). Catalyst systems that accomplish the coupling of sulfamates with a wide range of substrates are necessary to further broadening the synthetic utility of this protocol.
Figure 1.
Scope of metal-catalyzed amination of aryl sulfamates.
Here, we report a versatile Pd/phosphine catalyst for the amination of aryl sulfamates with a broad scope of N-nucleophiles, including anilines, primary and secondary alkyl amines, heteroaryl amines, N-heterocycles, and primary amides (Figure 1B). In addition, we provide evidence by DFT calculation that in the polar protic medium, a cationic pathway is operating.
Results and Discussion
In a previous work, we described the catalytic capability of a cationic N-methyl-2-aminobiphenyl palladacycle supported by sterically demanding phosphine PCyp2ArXyl2, 1, in the amination of aryl chlorides.11b The advantage of this precatalyst over the parent 2-aminobiphenyl palladacycle is that its activation in the presence of the base releases N-methyl carbazole, a byproduct that cannot hinder the catalytic reaction.13
Using precatalyst 1 (2.5 mol %), we examined the coupling of naphthalen-1-yl dimethylsulfamate with aniline applying the reaction conditions developed for the amination of aryl chlorides,11b namely, NaOtBu, as the base, dioxane as the solvent, and 110 °C as the reaction temperature. Under these conditions, a promising conversion to the expected N-phenylnaphthalen-1-amine, 4a, of 27% was observed by GC analysis (Table 1, entry 1). To further improve the yield, different solvents were investigated (entries 2–5). Either toluene or DMF provided lower conversions. The use of a protic solvent, such as tBuOH, which has been successfully applied in Pd-catalyzed C-N couplings,14 also resulted in lower yield of the product. It has been found that the addition of water can enhance the rate of the amination.14b,15 To our delight, the use of a 8:1 (vol.) mixture of tBuOH:H2O significantly increased the conversion, but for driving the reaction to completion, a 1:1 mixture of tBuOH and water was crucial, product 4a being isolated in 97% yield (entries 6–8; see also Table S1). Lowering the catalyst loading or using bases other than NaOtBu reduced the reaction efficiency (entries 9–11); in the latter case, decomposition of the naphthyl sulfamate into naphthol was observed. Notably, complete conversion was also achieved at temperatures as low as 60 °C (entry 12), and even at room temperature, 1 proved to be reactive giving product 4a in 74% yield (entry 13). However, a reaction temperature of 110 °C was used to study the reaction scope to accomplish the coupling of more challenging substrates.
Table 1. Screening of Conditions for the Coupling of Naphthalen-1-yl Dimethylsulfamate and Anilinea.
| entry | base | solvent | conversionb (%) |
|---|---|---|---|
| 1 | NaOtBu | dioxane | 27 |
| 2 | NaOtBu | THF | 12 |
| 3 | NaOtBu | toluene | 9 |
| 4 | NaOtBu | DMF | 15 |
| 5 | NaOtBu | tBuOH | 4 |
| 6 | NaOtBu | tBuOH:H2O (8:1) | 43 |
| 7 | NaOtBu | tBuOH:H2O (3:1) | 94 |
| 8 | NaOtBu | tBuOH:H2O (1:1) | 100 (97)c |
| 9d | NaOtBu | tBuOH:H2O (1:1) | 90 (85)c |
| 10 | LiOtBu | tBuOH:H2O (1:1) | 99 (91)c |
| 11 | NaOH | tBuOH:H2O (1:1) | 100 (90)c |
| 12e | NaOtBu | tBuOH:H2O (1:1) | 100 (92)c |
| 13f | NaOtBu | tBuOH:H2O (1:1) | (74)c |
Reaction conditions: naphthyl sulfamate (1 mmol), amine (1.2 mmol), base (1.2 mmol), 1 (0.025 mmol), solvent (2 mL), T = 110 °C, 18 h (unoptimized).
Conversion estimated by GC analysis of the reaction mixtures.
Yields of isolated products (average of two runs).
1 (0.020 mmol).
T = 60 °C.
Reaction performed at room temperature.
Using the optimized reaction conditions, we tested other ligands using the corresponding ligated N-methyl-2-aminobiphenyl palladacycles (see Table S2). Only with XPhos-supported palladacycle was the conversion comparable to that obtained with our precatalyst. However, precatalyst 1 outperformed the catalytic abilities of the XPhos-supported precatalyst when we examined the amination of other substrate combinations (see Table S3).
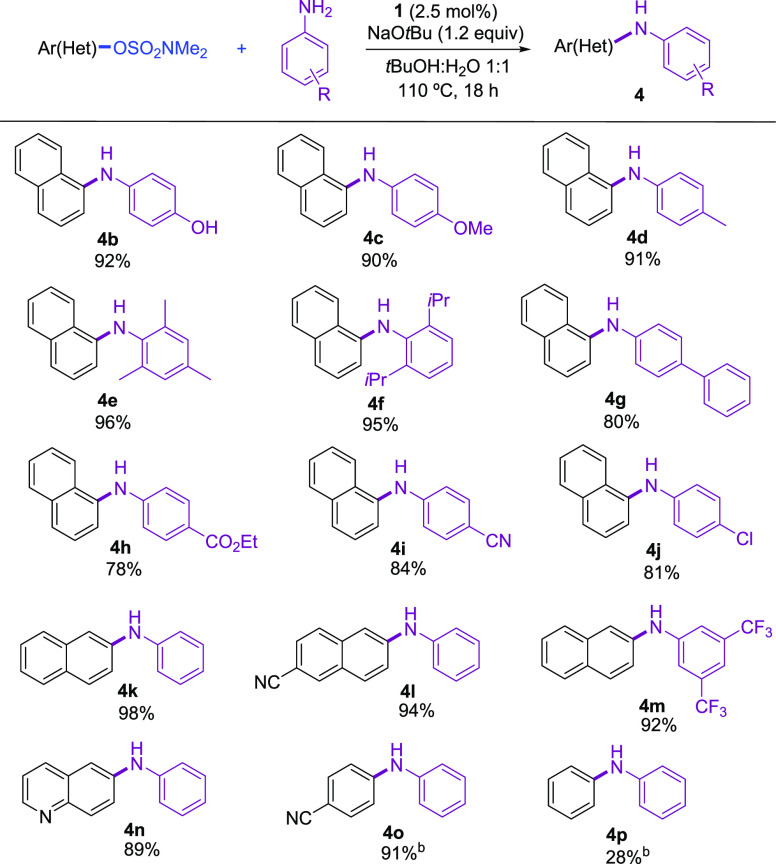
Under the optimized conditions, we examined the coupling of a variety of aryl sulfamates and anilines. As shown in Scheme 1, sulfamates derived from 1- and 2-naphthol could be successfully employed as reactant partners in this transformation (4b–4m). Furthermore, the catalytic system tolerated a quinoline core in the electrophile (4n). The nonfused ring p-cyanophenyl sulfamate could be efficiently coupled with aniline (4o); however, poor results were obtained with the less reactive phenyl sulfamate (4p; see Table S4). Regarding the aniline, the scope was broad. Both, electron-rich and electron-poor anilines provided the corresponding coupling products in yields higher than 81% (4b–4j, 4m). Moreover, ortho-substituted anilines were arylated in excellent yields (4e, 4f). Interestingly, naphthyl sulfamates could be selectively coupled with anilines bearing chloride (4j) or free hydroxyl functionalities (4b), avoiding the need of protecting groups or the use of weaker bases in the latter case.
Scheme 1. Pd-Catalyzed N-Arylation of Anilines with Aryl Sulfamates.
Reaction conditions: aryl sulfamate (1.0 mmol), amine (1.2 mmol), NaOtBu (1.2 mmol), 1 (0.025 mmol), solvent (2 mL), 110 °C, 18 h. Isolated yields of pure products.
Reaction performed with 3 mol % catalyst loading.
Next, the scope of aliphatic amines was explored (Scheme 2). Both cyclic and acyclic secondary amines proved to be suitable substrates furnishing the arylated products in useful synthetic yields, using the optimized reaction conditions (Scheme 2, 5a–h). Primary aliphatic amines have not been previously tested in Ni-catalyzed amination of aryl sulfamates. We found that our catalyst system enabled the coupling of benzylamine with naphthalen-2-yl sulfamate in high yield (5i). Moreover, linear alkyl primary amines could also be arylated with both naphthyl and phenyl-derived sulfamates, albeit in moderate yield (5j–5m).
Scheme 2. Pd-Catalyzed N-Arylation of Aliphatic Amines with Aryl Sulfamates.
Reaction conditions: aryl sulfamate (1.0 mmol), amine (1.2 mmol), NaOtBu (1.2 mmol), 1 (0.025 mmol), solvent (2 mL), 110 °C, 18 h. Isolated yields of pure products.
Conversion obtained using the Pd-XPhos precatalyst (Table S3).
4 mol % 1.
In light of the reactivity displayed by precatalyst 1 toward amines, we focused on more challenging N-nucleophiles like heteroarylamines and N-heterocycles, since they are present as substructures in biologically active molecules, natural products, and pharmaceuticals.12a,16 An array of substrate combinations were screened under the optimized conditions (Scheme 3). Regarding the heteroarylamines, 2- and 3-aminopyridine, 2-aminopyrimidine, and 2-aminopyrazine were successfully arylated providing the corresponding diarylamines in isolated yields ranging from 44 to 97% (Scheme 3, 6a–f). 2-Aminooxazol and 2-aminobenzoxazol were efficiently coupled with electron-rich and electron-deficient naphthyl sulfamate derivatives (6g, 6h). Pyridine methanamines were found compatible with the catalyst system, affording the N-arylated heterocycles in high yields (6i, 6j). Moreover, N-heterocycles such as pyrrole, pyrazole, and carbazole reacted with naphthalen-2-yl sulfamate, giving the desired cross-coupling products in moderate to high yields (Scheme 3, 7a–7c). Chemoselective N-arylation of indoles was efficiently accomplished (7d–f, 7h) although, with more hindered 2-substituted indoles, slightly lower yields were obtained (7g, 7i, 7j). To our knowledge, these results represent the first use of aryl sulfamates as electrophiles in the amination of a variety of N-nucleophiles that are relevant in pharmaceutical synthesis.
Scheme 3. Pd-Catalyzed N-Arylation of Heteroarylamines and N-Heterocycles with Aryl Sulfamates.
Reaction conditions: aryl sulfamate (1.0 mmol), amine (1.2 mmol), NaOtBu (1.2 mmol), 1 (0.025 mmol), solvent (2 mL), 110 °C, 18 h. Isolated yields of pure products.
Conversion obtained using the Pd-XPhos precatalyst (Table S3).
5 mol % 1.
Amides are problematic substrates in Pd-catalyzed C–N cross-coupling reactions, due to their reduced nucleophilicity together with their tendency to form k2-amidate complexes, responsible for retarding the rate of the reductive elimination step.17 However, despite these shortcomings, Pd-based catalysts have been developed that enable the N-arylation of amides even with C–O electrophiles,14b,18 albeit limited to the more reactive aryl sulfonates. We were pleased to find that parent benzamide and electron-rich and electron-poor benzamide derivatives were all effective N-arylated using the disclosed protocol (Scheme 4, 8a–d).
Scheme 4. Pd-Catalyzed N-Arylation of Amides with Aryl Sulfamatesa.
Reaction conditions: aryl sulfamate (1.0 mmol), amine (1.2 mmol), NaOtBu (1.2 mmol), 1 (0.035), solvent (2 mL), 110 °C, 18 h. Isolated yields of pure products.
Formamide proved to be more difficult resulting in modest yield of the desired product (8e). We are aware of a single example in which an aryl sulfamate was used as electrophile in a Ni-catalyzed N-arylation of primary amides.19
The catalytic cycle for the aryl amination reactions is well established20 and involves the three main steps common to any cross-coupling catalytic manifold: the oxidative addition, the ligand exchange (amine coordination and deprotonation), and the reductive elimination. Almost all the computational studies on C–N coupling reactions have been carried out with aryl halides as model electrophiles. To our knowledge, there is only one theoretical report, focused on the role of DBU as a base in C–N couplings with Pd/phosphine catalysts, in which a phenol-derived electrophile, p-tolyl triflate, is considered in the calculations.20h Bearing this in mind, we decided to explore the mechanism of the amination of aryl sulfamates with DFT calculations using (PCyp2ArXyl2)Pd(0) as the catalyst, naphthalen-1-yl dimethylsulfamate and aniline as the substrates, tBuO– and OH– as bases, and ethanol as the solvent, for the model chemical system. We used density functional methods (M06L/6-31g(d,p)/SDD//M06/6-311+g(2d,p)/LANL2TZ(f)).
We have shown that terphenyl phosphines can adopt pseudobidentate coordination modes, providing additional stabilization by noncovalent interactions between the metal center and a side ring of the terphenyl moiety.9a,10 Our calculations account for an extra stabilization of ca. −12 kcal mol–1 in ethanol for the PCyp2ArXyl2-ligated Pd(0) active species when the phosphine is binding in a pseudobidentate fashion (see Figure S1), in line with the results found for Nova et al.8d Therefore, calculations have been carried out considering only the bidentate coordination mode of the phosphine ligand. Thus, initial binding of the naphthyl sulfamates to the active Pd(0) species generates η2-naphthyl sulfamate complex A located −4.3 kcal mol–1 below the separate reactants in the free energy surface (Figure 2), which retains the bidentate coordination mode of the phosphine (k1-P,η2-Carene). Subsequently, oxidative addition at A is exergonic by 16.5 kcal mol–1 and gives rise to species B through an energy barrier of 27.3 kcal mol–1. This barrier, which is only 1.8 kcal mol–1 higher than that obtained by Nova et al.,8d in combination with the energy gain for the formation of B, renders this step not reversible. In accordance with the results obtained by Nova et al., in the oxidative addition product B, the sulfamate and the phosphine ligands display mutual trans orientation. Only one of the sulphonyl oxygen atoms of the sulfamate is bonded to the metal (Pd–O = 2.224 Å), and the phosphine retained the bidentate coordination (k1-P,η2-Carene), with the metrics for B being (Pd-C1′ = 2.511 Å, Pd-C2′ = 2.727 Å). To account for the lower reactivity observed for phenyl sulfamate derivatives, the oxidative addition of various p-substituted phenyl sulfamates (H, OH, CN) has been performed. Neutral or electron-donating substituents at the p-position of the aryl ring increase both the energy of the η2-sulfamate complex and the energy of the oxidative addition transition state, when compared with activated aryl sulfamates or naphthyl sulfamate (see Figure S2). This is consistent with our experimental observations.
Figure 2.
Gibbs free energy profile, in kcal mol–1, for the oxidative addition step.
For the ligand exchange step, first the oxidative addition intermediate B dissociates the sulfamate anion to give a T-shaped cationic complex C, where the position trans to the phosphine is vacant. This charge separation event is facilitated by polar solvents.21 In fact, calculations carried out with the continuum SMD model yielded energies of −19.8 and −26.1 kcal mol–1 for separate C and sulfamate in ethanol and water, respectively. However, the same charge separation event calculated in toluene results in the separate fragments lying +21.4 kcal mol–1 above the origin (Figure 3). These results support that polar protic solvent mixtures (ethanol and water) favor a dissociative pathway involving the formation of cationic complex C with retention of the secondary interaction with the terphenyl moiety, in line with the experimental observations (see Table 1, entry 6).
Figure 3.
Energy profile of the evaluated pathways for ligand exchange stage. Gibbs free energy profiles are in kcal mol–1.
Alternatively, the loss of the interaction between the metal and the flanking ring of the terphenyl substituent could also generate an open coordination site on the metal in intermediate B. However, the resulting species, D, which features k2-O coordination of the sulfamate is located 14.1 kcal mol–1 higher in energy than C (Figure 3). While D may be an accessible intermediate at the temperature at which reaction occurs, this pathway fails to explain the effect of the polar solvent mixture tBuOH:H2O in facilitating the reaction.
While aniline can coordinate to the metal center in the cationic intermediate C to yield the corresponding amino complex, other ligands present in the reaction mixture may also form intermediates worth considering in the analysis of the reaction mechanism (Figure 4). Consequently, we explored the coordination of aniline, water, OH–, and tBuO–.22
Figure 4.
Reaction pathways evaluated for ligand coordination to C. In parenthesis: Gibbs energy in kcal mol–1.
The coordination of aniline to C is thermoneutral (Figure 4), rendering cationic intermediate EPhNH2. Moreover, the association of H2O to intermediate C (Figure 4) yields intermediate EH2O with a relative Gibbs energy of −16.6 kcal mol–1, which is 3.2 kcal mol–1 higher than that of the aniline adduct EPhNH2. Therefore, H2O is not expected to be competitive with aniline for coordination to intermediate C. However, OH– coordinates to C to form neutral species EOH located at −37.0 below the reactants (Figure 4). Similarly, tBuO– forms a stable neutral adduct EOtBu but this species is present in very low concentrations (see below) in solution. The participation of the latter in the catalysis is deemed of little importance, and its discussion is relegated to the SI, while the role of the former is shown in the following paragraphs.
Following the above results, we envisioned two pathways for the ligand exchange step. The more favored one (shown in black in Figure 5) starts with cationic intermediate EPhNH2, which undergoes intermolecular deprotonation of the coordinated aniline by OH–. This step shows to be strongly favored thermodynamically (ΔG = −17.7 kcal mol–1), and importantly, the proton transfer occurs with a negligible energy barrier.23 The resulting species H, which displays the anilido ligand in the trans position to phosphorus, has a relative Gibbs energy of −37.1 kcal mol–1.
Figure 5.

Energy profile of the proposed pathways for the ligand exchange step and reductive elimination. Gibbs energies are in kcal mol–1.
On the contrary, in the alternative pathway (shown in green in Figure 5), OH– is acting as a ligand and as a base. From neutral intermediate EOH, coordination of aniline delivers intermediate FOH, located at −20.1 kcal mol–1. Intramolecular deprotonation of aniline is endergonic and takes place through TSFOH-GOH. The overall barrier from intermediate EOH is 28.6 kcal mol–1, slightly higher than that calculated for the oxidative step and higher than the reverse barrier for the dissociation of OH– in EOH to regenerate C. It is worth noting that all attempts to detect the corresponding Pd-hydroxo species experimentally were unsuccessful.
These considerations were further supported by application of microkinetic modeling. These analyses can give information about the evolution of the concentration of each species with time considering rate constants provided by DFT calculations.24 In our case, the microkinetic model indicated that the calculated barrier for oxidative addition may be overestimated by ca. 2 kcal mol–1 at 383 K and, more importantly, it revealed that EH2O, EOH, and EOtBu were present in very low concentration in the reaction mixture during the catalysis (see Figures S3 and S4 in the Supporting Information). In addition, the microkinetic model verified that the contribution of any pathway other than the one starting from intermediate EPhNH2 to the formation of the C–N coupling product is negligible.
Finally, the last stage of the catalytic cycle is the formation of the C–N bond from intermediate H. The reductive elimination takes place through TSH-I (Figure 5) with an activation energy barrier of 11.9 kcal·mol–1, generating intermediate I, in which the C–N product is coordinated to the Pd(0) center through the N atom. To close the catalytic cycle, displacement of the diarylamine by naphthalen-1-yl dimethylsulfamate occurs with an energy cost of 2.25 kcal mol–1.
Conclusions
In summary, we have developed a general Pd-based catalytic system for the amination of synthetically versatile aryl sulfamates. This protocol allows use of a broad range of N-nucleophiles including anilines, secondary amines, primary alkyl amines, heteroaryl amines, N-heterocycles, and primary amides. The use of a mixture of polar protic solvents (tBuOH:H2O) has been found to be crucial to attaining high conversions. Computational studies show that oxidative addition of aryl sulfamate is the rate-limiting step and that in polar protic solvents, the reaction proceeds through a cationic pathway.
Acknowledgments
We thank financial support from MCIN/AEI/10.13039/501100011033 (Grant PID2020-113797RB-C22), US/JUNTA/FEDER, UE (Grant US-1262266), and FEDER, UE/Junta de Andalucía-Consejería de Transformación, Economía, r Industria, Conocimiento y Universidades (Grant P20_00624) for financial support. A.M. thanks MICINN for a research fellowship. A.P. thanks Ministerio de Universidades (Plan de Recuperación Transformación y Resilencia) for financial support. The use of computational resources of the Universidad de Granada (cluster Albaicin) is thankfully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.3c03166.
General considerations; optimization experiments; unsuccessful results with aryl sulfamates; synthesis of aryl sulfamate substrates; general catalytic procedures; characterization data of reaction products; NMR spectra of compounds; computational details; microkinetic model (PDF)
Optimized coordinates for calculated structures (XYZ)
The authors declare no competing financial interest.
Supplementary Material
References
- a Qiu Z.; Li C.-J. Transformations of Less-Activated Phenols and Phenol Derivatives via C–O Cleavage. Chem. Rev. 2020, 120, 10454–10515. 10.1021/acs.chemrev.0c00088. [DOI] [PubMed] [Google Scholar]; b Zeng H.; Qiu Z.; Domínguez-Huerta A.; Hearne Z.; Chen Z.; Li C.-J. An Adventure in Sustainable Cross-Coupling of Phenols and Derivatives via Carbon–Oxygen Bond Cleavage. ACS Catal. 2017, 7, 510–519. 10.1021/acscatal.6b02964. [DOI] [Google Scholar]; c Cornella J.; Zarate C.; Martin R. Metal-catalyzed activation of ethers via C–O bond cleavage: a new strategy for molecular diversity. Chem. Soc. Rev. 2014, 43, 8081–8097. 10.1039/C4CS00206G. [DOI] [PubMed] [Google Scholar]
- For reviews see:; a Rosen B. M.; Quasdorft K. W.; Wilson D. A.; Zhang N.; Resmerita A.-M.; Garg N. K.; Percec V. Nickel-Catalyzed Cross-Couplings Involving Carbon–Oxygen Bonds. Chem. Rev. 2011, 111, 1346–1416. 10.1021/cr100259t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tobisu M.; Chatani N. Cross-Couplings Using Aryl Ethers via C–O Bond Activation Enabled by Nickel Catalysts. Acc. Chem. Res. 2015, 48, 1717–1726. 10.1021/acs.accounts.5b00051. [DOI] [PubMed] [Google Scholar]; c Boit T. B.; Bulger A. S.; Dander J. E.; Garg N. K. Activation of C–O and C–N Bonds Using Non-Precious-Metal Catalysis. ACS Catal. 2020, 10, 12109–12126. 10.1021/acscatal.0c03334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For recent examples on Ni-catalyzed C-O bond activation see:; a Lavoie C. M.; MacQueen P. M.; Rotta-Loria N. L.; Sawatzky R. S.; Borzenko A.; Chisholm A. J.; Hargreaves B. K. V.; McDonald R.; Ferguson M. J.; Stradiotto M. Challenging nickel-catalysed amine arylations enabled by tailored ancillary ligand design. Nat. Commun. 2016, 7, 11073. 10.1038/ncomms11073. [DOI] [PMC free article] [PubMed] [Google Scholar]; b MacQueen P. M.; Tassone J. P.; Diaz C.; Stradiotto M. Challenging nickel-catalysed amine arylations enabled by tailored ancillary ligand design. J. Am. Chem. Soc. 2018, 140, 5023–5027. 10.1021/jacs.8b01800. [DOI] [PubMed] [Google Scholar]; c Zhang J.; Sun T.; Zhang Z.; Cao H.; Bai Z.; Cao Z.-C. Nickel-Catalyzed Enantioselective Arylative Activation of Aromatic C–O Bond. J. Am. Chem. Soc. 2021, 143, 18380–18387. 10.1021/jacs.1c09797. [DOI] [PubMed] [Google Scholar]; d Borys A. M.; Hevia E. The Anionic Pathway in the Nickel-Catalysed Cross-Coupling of Aryl Ethers. Angew. Chem., Int. Ed. 2021, 60, 24659–24667. 10.1002/anie.202110785. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Day C. S.; Sommerville R. J.; Martin R. Deciphering the dichotomy exerted by Zn(II) in the catalytic sp2 C–O bond functionalization of aryl esters at the molecular level. Nat. Catal. 2021, 4, 124–133. 10.1038/s41929-020-00560-3. [DOI] [Google Scholar]
- For a review on Pd-catalyzed cross-coupling by C-O bond activation, see:; Zhou T.; Szostak M. Palladium-catalyzed cross-couplings by C–O bond activation. Catal. Sci. Technol. 2020, 10, 5702–5739. 10.1039/D0CY01159B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Snieckus V. Directed Ortho Metalation. Tertiary Amide and O-Carbamate Directors in Synthetic Strategies for Polysubstituted Aromatics. Chem. Rev. 1990, 90, 879–933. 10.1021/cr00104a001. [DOI] [Google Scholar]; b Macklin T. K.; Snieckus V. Directed Ortho Metalation Methodology. The N,N-Dialkyl Aryl O-Sulfamate as a New Directed Metalation Group and Cross-Coupling Partner for Grignard Reagents. Org. Lett. 2005, 7, 2519–2522. 10.1021/ol050393c. [DOI] [PubMed] [Google Scholar]; c Knappke C. E. I.; von Wangelin A. J. A Synthetic Double Punch: Suzuki–Miyaura Cross-Coupling Mates with C-H Functionalization. Angew. Chem., Int. Ed. 2010, 49, 3568–3570. 10.1002/anie.201001028. [DOI] [PubMed] [Google Scholar]
- a Bajo S.; Laidlaw G.; Kennedy A. R.; Sproules S.; Nelson D. J. Oxidative Addition of Aryl Electrophiles to a Prototypical Nickel (0) Complex: Mechanism and Structure/Reactivity Relationships. Organometallics 2017, 36, 1662–1672. 10.1021/acs.organomet.7b00208. [DOI] [Google Scholar]; b Uthayopas C.; Surawatanawong P. Aryl C-O oxidative addition of phenol derivatives to nickel supported by an N-heterocyclic carbene via a Ni0 five-centered complex. Dalton Trans. 2019, 48, 7817–7827. 10.1039/C9DT00455F. [DOI] [PubMed] [Google Scholar]
- a Rambren S. D.; Silberstein A. L.; Yang Y.; Garg N. K. Nickel-Catalyzed Amination of Aryl Sulfamates. Angew. Chem., Int. Ed. 2011, 50, 2171–2173. 10.1002/anie.201007325. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ackermann L.; Sandmann R.; Song W. Palladium- and Nickel-Catalyzed Aminations of Aryl Imidazolylsulfonates and Sulfamates. Org. Lett. 2011, 13, 1784–1786. 10.1021/ol200267b. [DOI] [PubMed] [Google Scholar]; c Hie L.; Ramgren S. D.; Mesganaw T.; Garg N. K. Nickel-Catalyzed Amination of Aryl Sulfamates and Carbamates Using an Air-Stable Precatalyst. Org. Lett. 2012, 14, 4182–4185. 10.1021/ol301847m. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Nathel N. F. F.; Kim J.; Hie L.; Jiang X.; Garg N. K. Nickel-Catalyzed Amination of Aryl Chlorides and Sulfamates in 2-Methyl-THF. ACS Catal. 2014, 4, 3289–3293. 10.1021/cs501045v. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Park N. H.; Teverovskiy G.; Buchwald S. L. Development of an Air-Stable Nickel Precatalyst for the Amination of Aryl Chlorides, Sulfamates, Mesylates, and Triflates. Org. Lett. 2014, 16, 220–223. 10.1021/ol403209k. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Inaloo I. D.; Majnooni S.; Eslahi H.; Esmaeilpour M. N-Arylation of (hetero)arylamines using aryl sulfamates and carbamates via C-O bond activation enabled by a reusable and durable nickel(0) catalyst. New J. Chem. 2020, 44, 13266–13278. 10.1039/D0NJ01610A. [DOI] [Google Scholar]
- a Molander G. A.; Shin I. Pd-Catalyzed Suzuki–Miyaura Cross-Coupling Reactions between Sulfamates and Potassium Boc-Protected Aminomethyltrifluoroborates. Org. Lett. 2013, 15, 2534–2537. 10.1021/ol401021x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang Z.-Y.; Ma Q.-N.; Li R.-H.; Shao L.-X. Palladium-catalyzed Suzuki–Miyaura coupling of aryl sulfamates with arylboronic acids. Org. Biomol. Chem. 2013, 11, 7899–7906. 10.1039/c3ob41382a. [DOI] [PubMed] [Google Scholar]; c Melvin P. R.; Hazari N.; Beromi M. M.; Shah H. P.; Williams M. J. Pd-Catalyzed Suzuki–Miyaura and Hiyama–Denmark Couplings of Aryl Sulfamates. Org. Lett. 2016, 18, 5784–5787. 10.1021/acs.orglett.6b02330. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Melvin P. R.; Nova A.; Balcells D.; Hazari N.; Tilset M. DFT Investigation of Suzuki–Miyaura Reactions with Aryl Sulfamates Using a Dialkylbiarylphosphine-Ligated Palladium Catalyst. Organometallics 2017, 36, 3664–3675. 10.1021/acs.organomet.7b00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Marín M.; Moreno J. J.; Navarro-Gilabert C.; Álvarez E.; Maya C.; Peloso R.; Nicasio M. C.; Carmona E. Synthesis, Structure and Nickel Carbonyl Complexes of Dialkylterphenyl Phosphines. Chem. – Eur. J. 2019, 25, 260–272. 10.1002/chem.201803598. [DOI] [PubMed] [Google Scholar]; b Marín M.; Moreno J. J.; Alcaide M. M.; Álvarez E.; López-Serrano J.; Campos J.; Nicasio M. C.; Carmona E. Evaluating Stereoelectronic Properties of Bulky Dialkylterphenyl Phosphine Ligands. J. Organomet. Chem. 2019, 896, 120–128. 10.1016/j.jorganchem.2019.06.003. [DOI] [Google Scholar]
- a Moreno J. J.; Espada M. F.; Campos J.; López-Serrano J.; Macgregor S. A.; Carmona E. Base-Promoted, Remote C–H Activation at a Cationic (η5-C5Me5)Ir(III) Center Involving Reversible C–C Bond Formation of Bound C5Me5. J. Am. Chem. Soc. 2019, 141, 2205–2210. 10.1021/jacs.8b11752. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Martín M. T.; Marín M.; Rama R. J.; Álvarez E.; Maya C.; Molina F.; Nicasio M. C. Zero-valent ML2 complexes of group 10 metals supported by terphenyl phosphanes. Chem. Commun. 2021, 57, 3083–3086. 10.1039/D1CC00676B. [DOI] [PubMed] [Google Scholar]
- a Rama R. J.; Maya C.; Nicasio M. C. Dialkylterphenyl Phosphine-Based Palladium Precatalysts for Efficient Aryl Amination of N-Nucleophiles. Chem. – Eur. J. 2020, 26, 1064–1073. 10.1002/chem.201903279. [DOI] [PubMed] [Google Scholar]; b Monti A.; Rama R. J.; Gómez B.; Maya C.; Álvarez E.; Carmona E.; Nicasio M. C. N-substituted aminobiphenyl palladacycles stabilized by dialkylterphenyl phosphanes: Preparation and applications in C–N cross-coupling reactions. Inorg. Chim. Acta 2021, 518, 120214 10.1016/j.ica.2020.120214. [DOI] [Google Scholar]
- a Ruiz-Castillo P.; Buchwald S. L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. 10.1021/acs.chemrev.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dorel R.; Grugel C. P.; Haydl A. M. The Buchwald–Hartwig Amination After 25 Years. Angew. Chem., Int. Ed. 2019, 58, 17118–17129. 10.1002/anie.201904795. [DOI] [PubMed] [Google Scholar]; c Rayadurgam J.; Sana S.; Sasikumar M.; Gu Q. Development of an Air-Stable Nickel Precatalyst for the Amination of Aryl Chlorides, Sulfamates, Mesylates, and Triflates. Org. Chem. Front. 2020, 8, 384–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama R. J.; Maya C.; Molina F.; Nova A.; Nicasio M. C. Important Role of NH-Carbazole in Aryl Amination Reactions Catalyzed by 2-Aminobiphenyl Palladacycles. ACS Catal. 2023, 13, 3934–3948. 10.1021/acscatal.3c00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See for example:; a Huang X.; Anderson K. W.; Zim D.; Jiang L.; Klapars A.; Buechwald S. L. Expanding Pd-Catalyzed C-N Bond-Forming Processes: The First Amidation of Aryl Sulfonates, Aqueous Amination, and Complementarity with Cu-Catalyzed Reactions. J. Am. Chem. Soc. 2003, 125, 6653–6655. 10.1021/ja035483w. [DOI] [PubMed] [Google Scholar]; b Fors B. P.; Dooleweerdt K.; Zeng Q.; Buchwald S. L. An Efficient System for the P-Catalyzed Cross-Coupling of Amides and Aryl Chlorides. Tetrahedron 2009, 65, 6576–6583. 10.1016/j.tet.2009.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kuwano R.; Utsunomiya M.; Hartwig J. F. Aqueous Hydroxide as a Base for Palladium-Catalyzed Amination of Aryl Chlorides and Bromides. J. Org. Chem. 2002, 67, 6479–6486. 10.1021/jo0258913. [DOI] [PubMed] [Google Scholar]; b Dallas A. S.; Gothelf K. V. Effect of Water on the Palladium-Catalyzed Amidation of Aryl Bromides. J. Org. Chem. 2005, 70, 3321–3323. 10.1021/jo0500176. [DOI] [PubMed] [Google Scholar]; c Lau S.-H.; Yu P.; Chen L.; Madsen-Duggan C. B.; Williams M. J.; Carrow B. P. Aryl Amination Using Soluble Weak Base Enabled by a Water-Assisted Mechanism. J. Am. Chem. Soc. 2020, 142, 20030–20039. 10.1021/jacs.0c09275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Magano J.; Dunetz J. Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. 10.1021/cr100346g. [DOI] [PubMed] [Google Scholar]; b McGowan M. A.; Henderson J. L.; Buchwald S. L. Palladium-Catalyzed N-Arylation of 2-Aminothiazoles. Org. Lett. 2012, 14, 1432–1435. 10.1021/ol300178j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K.; Yamashita M.; Puschmann F.; Alvarez-Falcon M. M.; Incarvito C. D.; Hartwig J. F. Organometallic Chemistry of Amidate Complexes. Accelerating Effect of Bidentate Ligands on the Reductive Elimination of N-Aryl Amidates from Palladium(II). J. Am. Chem. Soc. 2006, 128, 9044–9045. 10.1021/ja062333n. [DOI] [PubMed] [Google Scholar]
- a Yin J.; Buchwald S. L. Pd-Catalyzed Intermolecular Amidation of Aryl Halides: The Discovery that Xantphos Can Be Trans-Chelating in a Palladium Complex. J. Am. Chem. Soc. 2002, 124, 6043–6048. 10.1021/ja012610k. [DOI] [PubMed] [Google Scholar]; b Klapars A.; Campos K. R.; Chen C.-Y.; Volante R. P. Preparation of Enamides via Palladium-Catalyzed Amidation of Enol Tosylates. Org. Lett. 2005, 7, 1185–1188. 10.1021/ol050117y. [DOI] [PubMed] [Google Scholar]; c Dooleweerdt K.; Fors B. P.; Buchwald S. L. Pd-Catalyzed Cross-Coupling Reactions of Amides and Aryl Mesylates. Org. Lett. 2010, 12, 2350–2353. 10.1021/ol100720x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie C. M.; MacQueen P. M.; Stradiotto M. Nickel-Catalyzed N-Arylation of Primary Amides and Lactams with Activated (Hetero)aryl Electrophiles. Chem. – Eur. J. 2016, 22, 18752–18755. 10.1002/chem.201605095. [DOI] [PubMed] [Google Scholar]
- a Lombardi C.; Rucker R. P.; Froese R. D. J.; Sharif S.; Champagne P. A.; Organ M. G. Rate and Computational Studies for Pd-NHC-Catalyzed Amination with Primary Alkylamines and Secondary Anilines: Rationalizing Selectivity for Monoarylation versus Diarylation with NHC Ligands. Chem. – Eur. J. 2019, 25, 14223–14229. 10.1002/chem.201903362. [DOI] [PubMed] [Google Scholar]; b Sunesson Y.; Limé E.; Lill S. O. N.; Meadows R. E.; Norrby P.-O. Role of the Base in Buchwald–Hartwig Amination. J. Org. Chem. 2014, 79, 11961–11969. 10.1021/jo501817m. [DOI] [PubMed] [Google Scholar]; c Hoi K. H.; Çalimsiz S.; Froese R. D. J.; Hopkinson A. C.; Organ M. G. Amination with Pd–NHC Complexes: Rate and Computational Studies on the Effects of the Oxidative Addition Partner. Chem. – Eur. J. 2011, 17, 3086–3090. 10.1002/chem.201002988. [DOI] [PubMed] [Google Scholar]; d Hoi K. H.; Çalimsiz S.; Froese R. D. J.; Hopkinson A. C.; Organ M. G. Amination with Pd-NHC Complexes: Rate and Computational Studies Involving Substituted Aniline Substrates. Chem. – Eur. J. 2012, 18, 145–151. 10.1002/chem.201102428. [DOI] [PubMed] [Google Scholar]; e Cundari T. R.; Deng J. Density Functional Theory Study of Palladium-Catalyzed Aryl-Nitrogen and Aryl-Oxygen Bond Formation. J. Phys. Org. Chem. 2005, 18, 417–425. 10.1002/poc.889. [DOI] [Google Scholar]; f McMullin C. L.; Rühle B.; Besora M.; Orpen A. G.; Harvey J. N.; Fey N. Computational Study of PtBu3 as Ligand in the Palladium-Catalysed Amination of Phenylbromide with Morpholine. J. Mol. Catal. A: Chem. 2010, 324, 48–55. 10.1016/j.molcata.2010.02.030. [DOI] [Google Scholar]; g Yong F. F.; Mak A. M.; Wu W.; Sullivan M. B.; Robins E. G.; Johannes C. W.; Jong H.; Lim Y. H. Empirical and Computational Insights into N-Arylation Reactions Catalyzed by Palladium Meta -Terarylphosphine Catalyst. Chempluschem 2017, 82, 750–757. 10.1002/cplu.201700042. [DOI] [PubMed] [Google Scholar]; h Kim S.-T.; Pudasaini B.; Baik M.-H. Mechanism of Palladium-Catalyzed C–N Coupling with 1,8-Diazabicyclo[5.4.0]Undec-7-Ene (DBU) as a Base. ACS Catal. 2019, 9, 6851–6856. 10.1021/acscatal.9b02373. [DOI] [Google Scholar]; i Gómez-Orellana P.; Lledós A.; Ujaque G. Computational Analysis on the Pd-Catalyzed C–N Coupling of Ammonia with Aryl Bromides Using a Chelate Phosphine Ligand. J. Org. Chem. 2021, 86, 4007–4017. 10.1021/acs.joc.0c02865. [DOI] [PubMed] [Google Scholar]; j Ikawa T.; Barder T. E.; Biscoe M. R.; Buchwald S. L. Pd-Catalyzed Amidation of Aryl Chlorides Using Monodentate Biaryl Phosphine Ligands: A Kinetic, Computational, and Synthetic Investigation. J. Am. Chem. Soc. 2007, 129, 13001–13007. 10.1021/ja0717414. [DOI] [PubMed] [Google Scholar]; k Barder T. E.; Buchwald S. L. Insights into Amine Binding to Biaryl Phosphine Palladium Oxidative Addition Complexes and Reductive Elimination from Biaryl Phosphine Arylpalladium Amido Complexes via Density Functional Theory. J. Am. Chem. Soc. 2007, 129, 12003–12010. 10.1021/ja073747z. [DOI] [PubMed] [Google Scholar]
- Jutand A.; Mosleh A. Rate and Mechanism of Oxidative Addition of Aryl Triflates to Zerovalent Palladium Complexes. Evidence for the Formation of Cationic (σ-Aryl)palladium Complexes. Organometallics 1995, 14, 1810–1817. 10.1021/om00004a038. [DOI] [Google Scholar]
- McMullin C. L.; Jover J.; Harvey J. N; Fey N. Accurate modelling of Pd(0) + PhX oxidative addition kinetics. Dalton Trans. 2010, 39, 10833–10836. 10.1039/c0dt00778a. [DOI] [PubMed] [Google Scholar]
- Intermolecular deprotonation of EtNH2 shows an energy barrier of 2 kcal mol–1, see SI for details.
- a Besora M.; Maseras F. Microkinetic Modeling in Homogeneous Catalysis. WIREs Comput. Mol. Sci. 2018, 8, e1372. [Google Scholar]; b Jaraíz M.DFT-Based Microkinetic Simulations: A Bridge Between Experiment and Theory in Synthetic Chemistry. In Topics in Organometallic Chemistry; Springer Science and Business Media Deutschland GmbH, 2020; Vol. 67, pp. 81–105. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.