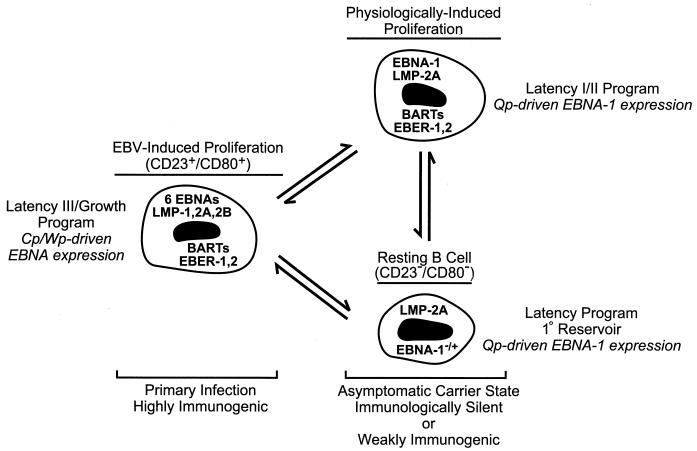
FIG. 1.
Model for the maintenance of EBV latency in B lymphocytes. In primary infection, a rapid EBV-induced expansion of infected B cells that express the full complement of known latency-associated genes (see text) serves to establish a pool of infected cells. These are equivalent to EBV-immortalized LCLs which characteristically express each of the six EBNA proteins through the Cp or Wp promoter, and they are susceptible to the developing cellular immunity directed toward EBV proteins expressed exclusively during type III latency (50). In the asymptomatic carrier state, resolution of primary infection is concomitant with the establishment of resting B cells as the primary reservoir of EBV in the peripheral blood (37, 38). These cells display a restricted pattern of EBV gene expression more characteristic of EBV-associated tumors such as BL (type I latency) or nasopharyngeal carcinoma (type II latency), in which Cp and Wp are silent and EBNA gene transcription is limited to EBNA-1 driven by the promoter Qp (6, 7, 9–11, 18, 22, 37, 45, 46, 53, 63, 67, 72, 75). Hypothetically, latently infected B cells are periodically subjected to EBV- or physiologically induced proliferation, e.g., in response to mitogenic signals such as CD40 ligand on activated T cells, that may serve to sustain a critical pool of infected B cells. Note that the precise pattern of EBV gene expression in the resting B cell is currently undefined, as expression of only LMP-2A and EBNA-1 has been evaluated for this population (37); however, studies of EBV gene expression in unfractionated B-lymphocyte populations (9, 10, 46, 72) have indicated that a broader pattern of expression including the BARTs and EBERs occurs in healthy carriers, and thus EBV gene expression in the resting B cell may be indistinguishable from that in the hypothetical infected B cell shown here that is proliferating in response to physiological signals.