Abstract
Background: People with multiple sclerosis (MS) and their physicians recognize cognitive retention as an important desired outcome of disease-modifying therapies (DMTs).
In this study, we attempted to gather the opinions of Iranian MS experts regarding the treatment approach toward clinical cases with different physical and cognitive conditions.
Methods: Opinions of 20 MS specialists regarding the best approach to 6 case scenarios (with different clinical, cognitive, and imaging characteristics) were gathered via a form.
Results: The estimated kappa of 0.16 [95% confidence interval (CI): 0.159-0.163; P < 0.001] suggested a poor degree of agreement on the treatment choice among the professionals.
Conclusion: Although most specialists agreed with treatment escalation in cases with cognitive impairment, there was no general agreement. Furthermore, there was not enough clinical evidence in the literature to develop consensus guidelines on the matter.
Key Words: Cognitive Dysfunction, Multiple Sclerosis, Treatment Escalation, Specialist, Iran
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease that causes significant disability in young adults. It is characterized pathologically by multifocal inflammatory lesions resulting in demyelination and variable degrees of axonal loss. 1 Progressive total brain volume (TBV) loss has been observed in all MS phenotypes,2-4 even in early relapsing-remitting MS (RRMS). 1 , 5,6 Brain atrophy shows the effect of irreversible MS pathophysiology, correlated with disability and cognitive dysfunction.6,7
MS has a wide range of focal neurological symptoms associated with psychological and cognitive manifestations.8,9 Its psychological and social burden on the patients is considerable. Cognitive impairment is one of its most common symptoms. It significantly impacts recent memory, attention, information processing speed, and executive function.10,11 Up to 60% of MS patients suffer from cognitive impairment, 12 which significantly impacts daily activities, work status,4 and family relationships.
In the last century, significant progress has been made in the pharmacological treatment of MS. There are a considerable variety of drugs with moderate to high potent effects on MS. Disease-modifying therapies (DMTs) reduce annualized relapse rate (ARR), lesion load in MRI, and neurologic disability measured by the Expanded Disability Status Scale (EDSS). 13 Second and third-line therapies such as natalizumab or alemtuzumab are significantly more effective on disability worsening and disease activity than first-line therapies such as β-interferon. 14 Although DMTs are effective in reducing the rate of MS disease progression, they cannot stop the neurodegenerative process completely.
People with MS and their physicians recognize cognitive retention as an important desired outcome of DMTs. 15 Some evidence points to the overall beneficial effect of a range of DMTs on cognitive function.16,17 There is no guideline indicating if the patient presents with symptoms or signs of cognitive impairment, their treatment course should be escalated to the next level DMTs. 18
In this study, we attempted to gather the opinions of Iranian MS experts regarding the treatment of clinical cases with different physical and cognitive conditions. Based on their responses, an attempt was made to present a basic general approach to dealing with similar cases.
Materials and Methods
In the present study, 6 case scenarios with different clinical, cognitive, and imaging characteristics were described in a form (Table 1). Data on demographic, academic, and clinical experiences of the participants were also added. The final version was made available online in Google form format. The link was shared in a WhatsApp group of Iranian MS specialists. All the members were academically trained and experienced neurologists in the field of MS.
Table 1.
Summary of the case scenarios
|
Case
No. |
Case description | Cognitive function | Physical status | MRI findings |
|---|---|---|---|---|
| 1 | 36 year-old female, known case of MS, on GA | Cognitive decline over 3 years | Stable | Stable |
| 2 | 41 year-old man, borderline cognitive function at MS diagnosis two years ago, on fingolimod | Severe cognitive decline in the last 6 months, other possible causes ruled out | Stable | New T2 lesions and severe cerebral atrophy |
| 3 | 45 year-old female, known case of MS, on IFN-beta 1a for 11 years | No previous cognitive evaluation, now shows impaired information processing speed (SDMT, PASAT: z score < -1.5) and delayed recall (CVLT-II delayed recall: z score < -1.5) | Stable | Cerebral atrophy |
| 4 | 32 year-old female, diagnosed with MS one year ago, on GA (plans for pregnancy) | Impaired information processing speed (SDMT: z score < -1.5), no change | Stable | Mild cortical atrophy at diagnosis, no change |
| 5 | 24 year-old male student, recently diagnosed with MS, IFN-beta 1a started, comes for the second opinion | Impaired information processing speed (SDMT, PASAT: z score < -1.5) (other causes ruled out) and delayed recall (CVLT-II delayed recall: z score < -1.5) | Right-sided hemiparesthesia (recovery with IVMP) | Brain MRI: 5 periventricular lesions (2 enhancing, 1 juxtacortical lesion) Spine MRI: normal |
| 6 | 38 year-old man, known case of MS for 3 years, on DMF | Evaluation not performed previously, now shows impaired information processing speed (SDMT, PASAT: z score < -1.5) | One mild non-disabling attack each year since then | Brain MRI: 1 enhancing periventricular lesion, otherwise stable Spine MRI: stable |
MS: Multiple Sclerosis; GA: Glatiramer acetate; MRI: Magnetic resonance imaging; SDMT: Symbol Digit Modalities Test; PASAT: Paced Auditory Serial Addition Test; CVLT-II: California Verbal Learning Test® Second Edition; IFN-beta: Interferon-β; DMF: Dimethyl fumarate; IVMP: Intravenous methylprednisolone
Cognitive impairment was defined as a score of less than -1.5 SD in two or more tests. 19
After checking for normality of the quantitative variables, descriptive analysis was performed. Correlations between demographic characteristics were assessed. The proportion of each selected option was calculated for each case. Moreover, Fleiss’ kappa was used to estimate the level of agreement among raters. 20
To assess the possible effect of experience on treatment approach, t-test was administered to compare mean age between those who preferred therapy escalation and those with preference for follow-up. SPSS (version 26, IBM Corp., Armonk, NY, USA) was used to analyze the data. P-values of less than 0.05 were considered statistically significant.
The study protocol was approved by the ethics committee of Tehran University of Medical Sciences, Iran, with the IRB code “IR.TUMS.NI.REC.1400.069”.
Results
All the members participated in over 2 days of link sharing. The demographic characteristics of the participants are summarized in table 2.
Table 2.
The demographic characteristics of the participants
| Variable | Value |
|---|---|
| Age (year) (mean ± SD) | 43.3 ± 6.1 |
| Gender [n (%)] | |
| Female | 10 (45.5) |
| Male | 12 (54.5) |
| Years of practice in the field of MS (mean ± SD) | 8.0 ± 6.1 |
| Estimated average number of MS patients visited in a month [interquartile range (IQR)] | 250 (100-400) |
MS: Multiple Sclerosis; SD: Standard deviation
Mean ± SD for quantitative variables with normal distribution, median [interquartile range (IQR)] for quantitative variables without normal distribution, and number (percentage) for qualitative variables.
Among the quantitative variables, the distribution of the “estimated average number of MS patients visited in a month” did not seem to be normal.
As predicted, age perfectly correlated with years of practice (r = 0.8; P < 0.001); female specialists were significantly younger than male specialists (mean difference: 8.0 years; P = 0.001).
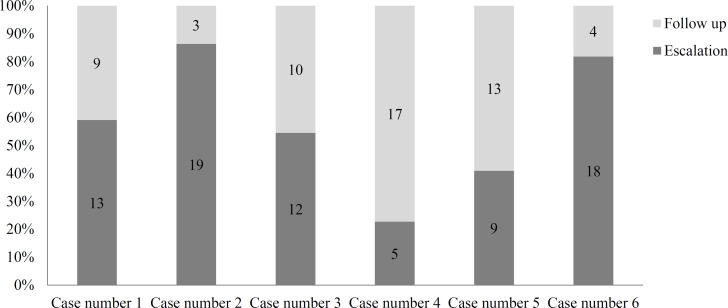
The treatment approach for each clinical scenario is presented in figure 1. Regarding case 1 (who was reported to experience cognitive decline over 3 years in the absence of other clinical or imaging features of disease activity), 13 (59.1%) specialists believed that the DMT should be escalated. Checking for possible associations, we found that those in favor of escalation were more experienced (mean difference = 5.7, P = 0.020).
Only 3 (13.6%) of the participants preferred follow-up for case 2 who presented with severe cognitive decline in a relatively short period in addition to new MRI lesions and atrophy, with no other clinical evidence of disease activity.
Figure 1.
100% stacked bar charts showing variability of opinions concerning the treatment approach for the clinical scenarios
However, 2 of these 3 individuals also recommended cognitive rehabilitation. No association was found between any basic characteristics of these professionals and their approach (P > 0.050).
Among the respondents, 12 (54.5%) believed that escalation was required for case 3 (who has had no previous cognitive evaluation, was recently diagnosed with cognitive impairment, had cerebral atrophy, and had no other physical problems). No association was found between the participants’ characteristics and the chosen option (P > 0.050).
The majority of specialists (17/22; 77.3%) thought that there is no need to change the treatment for case 4 (who indicated stable borderline cognitive impairment, stable mild cortical atrophy, and no other clinical problems). The respondents’ characteristics did not show any association with their approach (P > 0.050).
Follow-up was the chosen approach for case 5 by 13 (59.1%) participants, without any significant association with their characteristics (P > 0.050).
The 4 professionals (18.2%) who preferred escalation for case 6 (who indicated recurrent mild attacks with significant cognitive impairment) were significantly younger (mean difference: 12.5; P < 0.001).
The estimated kappa of 0.16 [95% confidence interval (CI): 0.159-0.163; P < 0.001] suggested a poor degree of agreement on the treatment choice among professionals. 21
Discussion
This study investigated the variability of experts’ opinions concerning the treatment approaches for MS patients. In the dilemma of choosing between follow-up and escalation in approaching cognitive impairment in MS patients, our surveyed professionals were in disagreement on which therapy to use. In fact, there are no clear guidelines for determining the best approach as yet and different patient conditions make decision-making more difficult for physicians. Our case scenarios were designed to identify the different clinical aspects of the topic by challenging specialists.
The literature review did not reveal any agreement between the studies in choosing the best approach for treatment. However, conventional approaches in any center or country are in accordance with the epidemiological pattern of patients referring to specialists and receiving a treatment based on the approach followed in that center and the available facilities. In a study similar to ours, Almusalam et al. compared 195 neurologists, experts in MS, in different countries.22 They investigated clinical decisions of experts based on 10 simulated MS case scenarios. They sought the prevalence of clinical inertia, defined as resistance to indicated therapeutic escalation. The results suggested a paucity of therapy escalation. According to their findings, the incidence and intensity of inertia were the lowest in Canadian individuals. Higher inertia ratings were linked to a lower level of MS care knowledge and a greater aversion to ambiguity. 22 In another study conducted among neurologists of the Spanish Society of Neurology, it was concluded that due to disagreements among neurologists on the type of treatment, a new consensus statement must be drafted. 23 Another similarpanel of experts in Argentina also changed their previous consensus guidelines of MS care. 24
To the best of our knowledge, none of the previous studies have surveyed physicians’ opinions on the treatment approach for cognitive impairment; however, the review of the clinical evidence should be considered along with experts’ opinions for any consensus recommendations.
The results showed that, in most cases, specialists suggested therapy escalation based on the patients’ clinical condition. Notwithstanding, there was no general agreement on therapy escalation for patients based on specialists’ opinions. A study by Utz et al. revealed that cognitive impairment was stable during 1 year of treatment for patients undergoing DMT. 25 In another survey, Landmeyer et al. found that DMT improved the clinical course of cognitive impairment.26 Nevertheless, there was no evidence that patients required therapy escalation. 26 Therefore, the evaluation of cognitive situations in MS patients should be further discussed and evaluated by clinicians using neuroimaging. 27 In this regard, all of our simulated case scenarios were assessed by MRI and cognitive tests. However, presently, despite numerous suggestions and factors for therapy escalation, these assessments are not included in the Canadian MS working group, RIO, and modified RIO, which are used as criteria for escalated patients. In particular, the Canadian MS Working Group recommends that a change in DMT should be considered for patients who meet any of the major criteria including more than 2 relapses in the first year of treatment, moderate to severe clinical symptoms, functional impairment, motor/cerebellar/brain stem/sphincter involvement, lack of complete recovery, EDSS change of more than 1 point after 6 months, and more than 3 new lesions on MRI during treatment. 28 In addition to the above criteria, the RIO score (from 0 to 3) is also used for decision-making regarding therapy escalation. The RIO score criteria include disease progression (EDSS), active MRI lesions, and recurrence of the disease in the first year of treatment. 29 The score increases with the appearance of some symptoms such as lesions on MRI results, relapses, and disease progression within 6 months. When there are signs of disease progression and relapse in the patient during 12 months, a score of greater than 2 is considered. 30 The modified RIO score (MRS) includes the combination of MRI activity and clinical relapses. 29 In the new RIO score, the score varies from 0 to 2. A patient who has an excellent response to treatment obtains a score of 0, and the patient who does not respond to treatment received a score of 2. A patient with a score of 1 is followed for 6 months. The observing of recurrence or lesions on MRI results is considered as non-response to treatment.31,32 Regarding all the mentioned criteria, our simulated case 1, who was under treatment with GA, was not an escalation candidate based on the RIO, modified RIO, and Canadian group criteria, as the patient had no experience of relapse, disability progression, or MRI activity. However, 13 specialists (59.1%) agreed with the drug change based on the progressive cognitive impairment over the last 3 years. This case was an example indicating the need for a new strategy for the evaluation of complicated patients.
Our simulated case scenarios were designed in a way would challenge acting experts in the selection of escalation or follow-up based on the available criteria of RIO or modified RIO. Our second case scenario, as a good example, was an MS patient who had borderline cognitive function at the time of MS diagnosis 2 years ago, and had been treated with fingolimod during these 2 years. Although her physical status has been stable, she has had severe cognitive decline over the last 6 months. New T2 lesions and severe cerebral atrophy were the findings of imaging studies. Experts preferred therapy escalation, but there was no indication for escalation in the previously mentioned recommendations. Similarly, case 3 indicated cognitive impairment and cerebral atrophy after 11 years of treatment with IFN-beta 1. The experts preferred therapy escalation in contrast to the RIO criteria. We also tried to include much more complicated cases in the simulation, such as case 4, who was properly treated with glatiramer acetate for 1 year and was planning to get pregnant. New cognition tests and MRI findings showed no significant changes; however, mild cognitive impairment and mild cortical atrophy were present at baseline. Most of the experts preferred follow-up in line with the follow-up recommendation of RIO. Case 5 experienced right-sided hemiparesthesia attack and was improved by IV methylprednisolone. Physical and brain MRI findings of periventricular lesions were not indicative of a need for changing the treatment; however, most experts preferred follow-up with moderate-efficacy DMTs than early intensive treatment (EIT), even though the patient suffered significant cognitive impairment. Most experts preferred therapy escalation for a patient (case 6) who was treated with DMF and showed cognitive impairment after 3 years. Recurrent mild attacks with significant cognitive impairment were the reason for preferring therapy escalation.
A review conducted in 2016 revealed the concordance of MRI findings and cognitive impairment with focal or diffuse damage in some areas of the brain. 33 Our study indicated cerebral atrophy in cases 2, 3, and 4. Moreover, early effective treatment strategies and the effect of some treatments on cognitive impairment (inconsistent with our study results) have been discussed previously. For example, the findings of Johnen et al. confirmed the effect of appropriate treatment on preventing cognitive impairments in pediatric patients with MS. 34 Most neurologists believe that only relapses indicate the failure of the treatment strategy. Therefore, the necessity of using therapy escalation to prevent or halt cognitive dysfunction should be discussed and evaluated in future studies. 35
Iaffaldano et al. studied 2702 patients to evaluate long-term disability after EIT and escalation followed by moderate-efficacy to high-efficacy DMTs [escalation treatment (ESC)]. They indicated that EIT over time is more effective than controlling disability or cognitive impairment through ESC strategy, suggesting the necessity of long-term follow-up for our escalated patients. 36 Moreover, in their study, the ESC group received glatiramer acetate similar to case 1 in our study. Harding et al. conducted a comparative study to analyze clinical outcomes of EIT versus escalation in patients with MS. 37 Their study showed that the long-term effects of EIT were more favorable than using moderate efficacy DMTs as the first line treatment. However, our study participants did not suggest this strategy for a 24-year-old male student with impaired cognitive function and MRI activity at baseline. It must be noted that clinicians must evaluate the treatment pathway based on the severity of the disease, long-term safety, pregnancy (like case 4 in our study) or the patient’s plan for pregnancy, side effects, and individual factors. 38 Furthermore, in a cohort study, the collected data on 2 national strategies for treating RRMS indicated the advantage of therapy escalation over the initial treatment in cases with disability. 39 An observational study concluded that treatment escalation leads to fewer relapses. 40 Case 6 in our study was a patient under treatment with DMF. DMF is one of the first-line agents approved by the US Food and Drug Administration (FDA) in 2013, and was initially used for psoriasis. The mechanism is now considered a proper first-line therapy for MS as it activates the nuclear-related factor 2 transcriptional pathway and has the potential to reduce oxidative stress. 41 However, some conditions like cognitive impairments in our cases led the specialists to escalate the treatment, though the Canadian MS working group, RIO, and modified RIO have not recommended escalation.
It should be noted that the specialists participating in this study were not at the same level in terms of experience in the treatment of MS patients; some specialists were more experienced than others. Specialists who had more experience in treating MS patients were more likely to agree with patient escalation than others.
Limitations: There are some limitations to our study. First, the small number of participants could have skewed the results. Second, clinicians’ decisions to escalate treatment or perform a follow-up could be affected by factors such as drug availability, local legislation, drug costs, and differences in clinical circumstances related to different patients.
Conclusion
Finally, although most specialists agreed with therapy escalation, there was no general agreement that cognitive impairment in patients could be treated through therapy escalation, and there was not enough clinical evidence in the literature to develop consensus guidelines on this matter. Therefore, further clinical trials are required to investigate the effectiveness of therapy escalation in patients with cognitive impairment.
Acknowledgments
This study was funded by Tehran University of Medical Sciences (grant number: 1400-3-233-56038).
Notes:
How to cite this article: Ghadiri F, Asadollahzadeh E, Ebadi Z, Sahraian MA, Azimi A, Navardi S, et al. Iranian specialists’ approach to treatment escalation in multiple sclerosis patients with cognitive impairment. Curr J Neurol 2023; 22(1): 1-7.
Conflict of Interests
The authors declare no conflict of interest in this study.
References
- 1.Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Bruck W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125(Pt 10):2202–12. doi: 10.1093/brain/awf235. [DOI] [PubMed] [Google Scholar]
- 2.Rudick RA, Fisher E, Lee JC, Simon J, Jacobs L. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology. 1999;53(8):1698–704. doi: 10.1212/wnl.53.8.1698. [DOI] [PubMed] [Google Scholar]
- 3.Losseff NA, Wang L, Lai HM, Yoo DS, Gawne-Cain ML, McDonald WI, et al. Progressive cerebral atrophy in multiple sclerosis. A serial MRI study. Brain. 1996;119(Pt 6):2009–19. doi: 10.1093/brain/119.6.2009. [DOI] [PubMed] [Google Scholar]
- 4.Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17(1):479–89. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 5.Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: A longitudinal study. Ann Neurol. 2008;64(3):255–65. doi: 10.1002/ana.21436. [DOI] [PubMed] [Google Scholar]
- 6.Chard DT, Griffin CM, Parker GJ, Kapoor R, Thompson AJ, Miller DH. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125(Pt 2):327–37. doi: 10.1093/brain/awf025. [DOI] [PubMed] [Google Scholar]
- 7.Filippi M, Rocca MA. MR imaging of gray matter involvement in multiple sclerosis: implications for understanding disease pathophysiology and monitoring treatment efficacy. AJNR Am J Neuroradiol. 2010;31(7):1171–7. doi: 10.3174/ajnr.A1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–17. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 9.Blaszczyk JW, Cieslinska-Swider J, Orawiec R. New methods of posturographic data analysis may improve the diagnostic value of static posturography in multiple sclerosis. Heliyon. 2021;7(2):e06190. doi: 10.1016/j.heliyon.2021.e06190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7(12):1139–51. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 11.Amato MP, Zipoli V, Portaccio E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci. 2006;245(1-2):41–6. doi: 10.1016/j.jns.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Planche V, Gibelin M, Cregut D, Pereira B, Clavelou P. Cognitive impairment in a population-based study of patients with multiple sclerosis: Differences between late relapsing-remitting, secondary progressive and primary progressive multiple sclerosis. Eur J Neurol. 2016;23(2):282–9. doi: 10.1111/ene.12715. [DOI] [PubMed] [Google Scholar]
- 13.Fogarty E, Schmitz S, Tubridy N, Walsh C, Barry M. Comparative efficacy of disease-modifying therapies for patients with relapsing remitting multiple sclerosis: Systematic review and network meta-analysis. Mult Scler Relat Disord. 2016;9:23–30. doi: 10.1016/j.msard.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Kalincik T, Brown JWL, Robertson N, Willis M, Scolding N, Rice CM, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: A cohort study. Lancet Neurol. 2017;16(4):271–81. doi: 10.1016/S1474-4422(17)30007-8. [DOI] [PubMed] [Google Scholar]
- 15.Day GS, Rae-Grant A, Armstrong MJ, Pringsheim T, Cofield SS, Marrie RA. Identifying priority outcomes that influence selection of disease-modifying therapies in MS. Neurol Clin Pract. 2018;8(3):179–85. doi: 10.1212/CPJ.0000000000000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riepl E, Pfeuffer S, Ruck T, Lohmann H, Wiendl H, Meuth SG, et al. Alemtuzumab improves cognitive processing speed in active multiple sclerosis-a longitudinal observational study. Front Neurol. 2017;8:730. doi: 10.3389/fneur.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muckschel M, Beste C, Ziemssen T. Immunomodulatory treatments and cognition in MS. Acta Neurol Scand. 2016;134 (Suppl 200):55–9. doi: 10.1111/ane.12656. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benedict RH, Cookfair D, Gavett R, Gunther M, Munschauer F, Garg N, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS) J Int Neuropsychol Soc. 2006;12(4):549–58. doi: 10.1017/s1355617706060723. [DOI] [PubMed] [Google Scholar]
- 20.Davies M, Fleiss JL. Measuring agreement for multinomial data. Biometrics. 1982;38(4):1047–51. [Google Scholar]
- 21.Altman DG. Practical statistics for medical research. Boca Raton, FL: CRC Press; 1990. [Google Scholar]
- 22.Almusalam N, Oh J, Terzaghi M, Maurino J, Bakdache F, Montoya A, et al. Comparison of physician therapeutic inertia for management of patients with multiple sclerosis in Canada, Argentina, Chile, and Spain. JAMA Netw Open. 2019;2(7):e197093. doi: 10.1001/jamanetworkopen.2019.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia MA, Ramon Ara CJ, Fernandez FO, Landete PL, Moral TE, Rodriguez-Antiguedad ZA. Consensus statement on the treatment of multiple sclerosis by the Spanish Society of Neurology in 2016. Neurologia. 2017;32(2):113–9. doi: 10.1016/j.nrl.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Cristiano E, Alonso R, Alvez PA, Bacile EA, Balbuena ME, Ballario C, et al. Argentinean recommendations on the identification of treatment failure in relapsing remitting multiple sclerosis patients. J Neurol Sci. 2018;385:217–24. doi: 10.1016/j.jns.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Utz KS, Lee DH, Lammer A, Waschbisch A, Linker RA, Schenk T. Cognitive functions over the course of 1 year in multiple sclerosis patients treated with disease modifying therapies. Ther Adv Neurol Disord. 2016;9(4):269–80. doi: 10.1177/1756285616643892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landmeyer NC, Burkner PC, Wiendl H, Ruck T, Hartung HP, Holling H, et al. Disease-modifying treatments and cognition in relapsing-remitting multiple sclerosis: A meta-analysis. Neurology. 2020;94(22):e2373–e2383. doi: 10.1212/WNL.0000000000009522. [DOI] [PubMed] [Google Scholar]
- 27.Pflugshaupt T, Geisseler O, Nyffeler T, Linnebank M. Cognitive impairment in multiple sclerosis: clinical manifestation, neuroimaging correlates, and treatment. Semin Neurol. 2016;36(2):203–11. doi: 10.1055/s-0036-1579696. [DOI] [PubMed] [Google Scholar]
- 28.Freedman MS, Devonshire V, Duquette P, Giacomini PS, Giuliani F, Levin MC, et al. Treatment optimization in multiple sclerosis: Canadian MS Working Group Recommendations. Can J Neurol Sci. 2020;47(4):437–55. doi: 10.1017/cjn.2020.66. [DOI] [PubMed] [Google Scholar]
- 29.Jamroz-Wisniewska A, Zajdel R, Slowik A, Marona M, Wnuk M, Adamczyk-Sowa M, et al. Modified Rio score with platform therapy predicts treatment success with fingolimod and natalizumab in Relapsing-Remitting Multiple Sclerosis Patients. J Clin Med. 2021;10(9):1830. doi: 10.3390/jcm10091830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith AL, Cohen JA, Hua LH. Therapeutic targets for multiple sclerosis: current treatment goals and future directions. Neurotherapeutics. 2017;14(4):952–60. doi: 10.1007/s13311-017-0548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nixon R, Bergvall N, Tomic D, Sfikas N, Cutter G, Giovannoni G. No evidence of disease activity: Indirect comparisons of oral therapies for the treatment of relapsing-remitting multiple sclerosis. Adv Ther. 2014;31(11):1134–54. doi: 10.1007/s12325-014-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kappos L, De SN, Freedman MS, Cree BA, Radue EW, Sprenger T, et al. Inclusion of brain volume loss in a revised measure of 'no evidence of disease activity' (NEDA-4) in relapsing-remitting multiple sclerosis. Mult Scler. 2016;22(10):1297–305. doi: 10.1177/1352458515616701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paul F. Pathology and MRI: Exploring cognitive impairment in MS. Acta Neurol Scand. 2016;134 (Suppl 200):24–33. doi: 10.1111/ane.12649. [DOI] [PubMed] [Google Scholar]
- 34.Johnen A, Elpers C, Riepl E, Landmeyer NC, Kramer J, Polzer P, et al. Early effective treatment may protect from cognitive decline in paediatric multiple sclerosis. Eur J Paediatr Neurol. 2019;23(6):783–91. doi: 10.1016/j.ejpn.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Ontaneda D, Tallantyre E, Kalincik T, Planchon SM, Evangelou N. Early highly effective versus escalation treatment approaches in relapsing multiple sclerosis. Lancet Neurol. 2019;18(10):973–80. doi: 10.1016/S1474-4422(19)30151-6. [DOI] [PubMed] [Google Scholar]
- 36.Iaffaldano P, Lucisano G, Caputo F, Paolicelli D, Patti F, Zaffaroni M, et al. Long-term disability trajectories in relapsing multiple sclerosis patients treated with early intensive or escalation treatment strategies. Ther Adv Neurol Disord. 2021;14:17562864211019574. doi: 10.1177/17562864211019574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harding K, Williams O, Willis M, Hrastelj J, Rimmer A, Joseph F, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019;76(5):536–41. doi: 10.1001/jamaneurol.2018.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vargas DL, Tyor WR. Update on disease-modifying therapies for multiple sclerosis. J Investig Med. 2017;65(5):883–91. doi: 10.1136/jim-2016-000339. [DOI] [PubMed] [Google Scholar]
- 39.Spelman T, Magyari M, Piehl F, Svenningsson A, Rasmussen PV, Kant M, et al. treatment escalation vs immediate initiation of highly effective treatment for patients with relapsing-remitting multiple sclerosis: Data from 2 different national strategies. JAMA Neurol. 2021;78(10):1197–204. doi: 10.1001/jamaneurol.2021.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chalmer TA, Kalincik T, Laursen B, Sorensen PS, Magyari M. Treatment escalation leads to fewer relapses compared with switching to another moderately effective therapy. J Neurol. 2019;266(2):306–15. doi: 10.1007/s00415-018-9126-y. [DOI] [PubMed] [Google Scholar]
- 41.Linker RA, Gold R. Dimethyl fumarate for treatment of multiple sclerosis: mechanism of action, effectiveness, and side effects. Curr Neurol Neurosci Rep. 2013;13(11):394. doi: 10.1007/s11910-013-0394-8. [DOI] [PubMed] [Google Scholar]