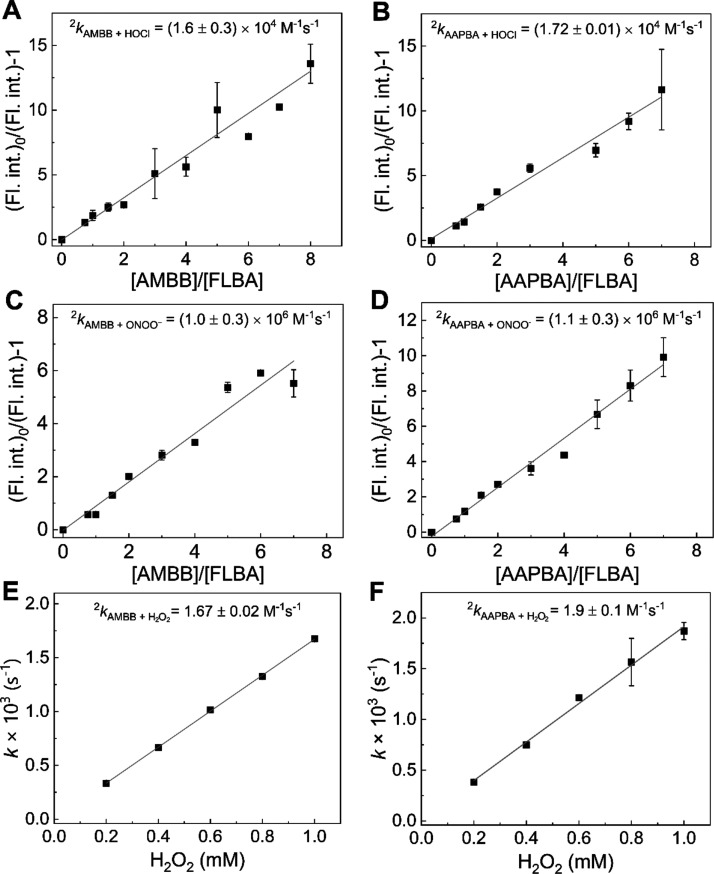
Figure 6.
Kinetics of the reaction between the boronate derivatives of acetaminophen and HOCl, ONOO–, and H2O2, determined at pH 7.4. (A) Relationship used to determine the rate constant between AMBB and HOCl according to the competition kinetic approach. Incubation mixtures contained 20 μM FLBA, 15–160 μM AMBB, 1.5 μM NaOCl, 50 mM phosphate buffer (pH 7.4), and 5% (v/v) ACN. (B) Same as (A) but incubation mixtures contained AAPBA instead of AMBB. (C) Same as (A) but incubation mixtures contained 1.5 μM ONOO– instead of NaOCl. (D) Same as (B) but incubation mixtures contained 1.5 μM ONOO– instead of NaOCl. (E) Dependence of pseudo-first-order rate constant of AMBB oxidation on a H2O2 concentration. Incubation mixtures contained 0.2–1 mM H2O2, 20 μM AMBB, 20 mM phosphate buffer (pH 7.4), and 2.5% (v/v) ACN. (F) Same as (E) but incubation mixtures contained AAPBA instead of AMBB. The solid lines represent the linear fittings according to the presented equation (see the Experimental Procedures section). Points represent means ± S.D. for at least two independent measurements.