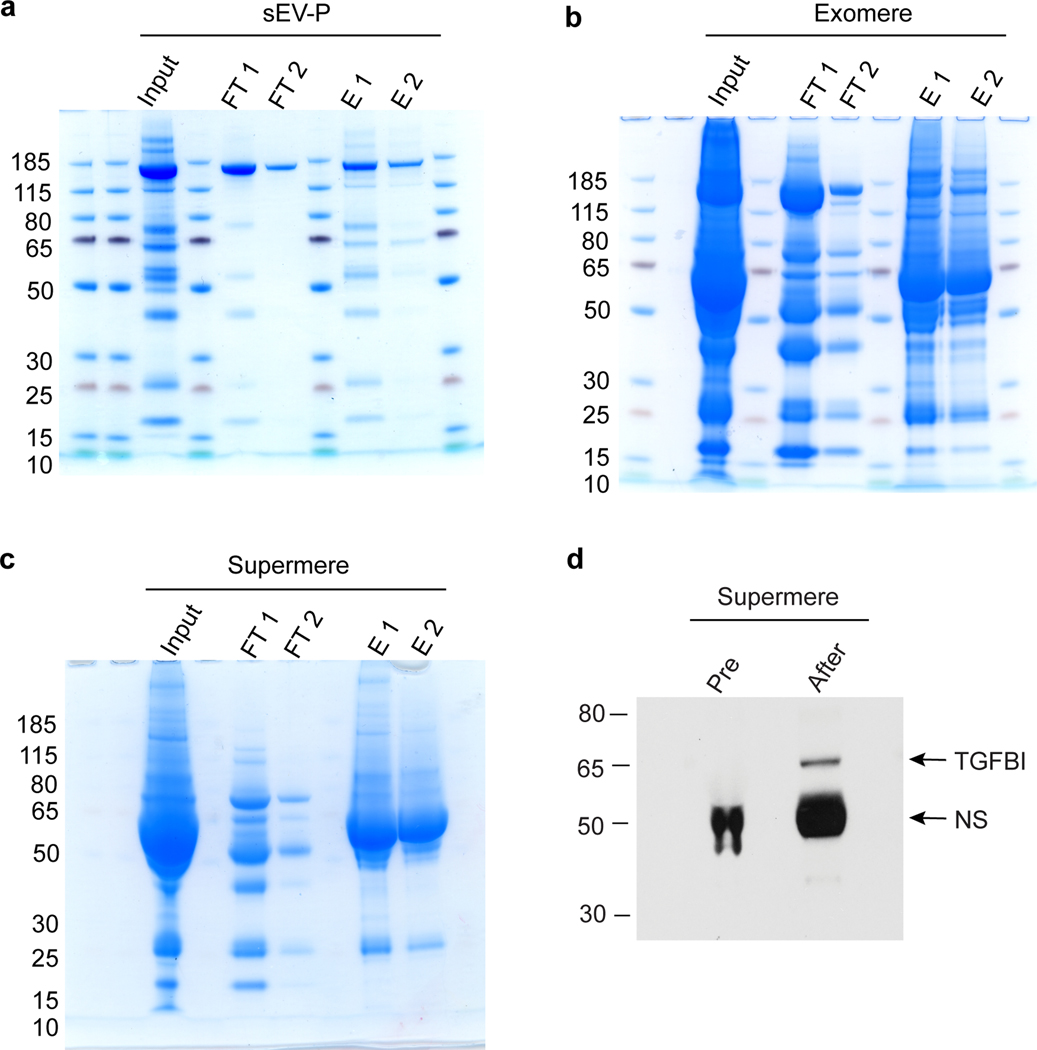
Fig. 7. Depletion of albumin from sEV-Ps, exomeres and supermeres isolated from CRC patient plasma.
The extracellular samples are isolated from a CRC patient plasma as described in the protocol (Box 3) with concentrations of 4.8, 78.3 and 83.3 μg/μl proteins for sEV-Ps, exomeres and supermeres respectively. Equal volumes (40 μl) of each fraction are diluted 5-fold in albumin binding buffer and applied to the column for albumin depletion using the Albumin Depletion Kit (Abcam) as described in the protocol (Box 3). Equal volume (12 μl) of input (pre-albumin depletion), albumin depleted samples (two flow-throughs, FT1 and FT2) and eluted albumin samples (two elutions, E1 and E2, mainly containing albumin) are analyzed by 4–12% SDS-PAGE and stained by Coomassie blue. a, albumin depletion from sEV-Ps. b, albumin depletion from exomeres. c, albumin depletion from supermeres. d, Immunoblot analysis of TGFBI levels in albumin-depleted supermeres derived from colorectal cancer patient plasma. Albumin was depleted in supermeres as described above, and analyzed by immunoblotting for TGFBI expression. Equal amount (30 μg) of pre- and after albumin-depleted supermeres are used for immunoblot analysis with the antibodies indicated. Research conducted as part of this protocol complies with all the relevant ethical regulations. The use of the human samples was approved by the Vanderbilt University Medical Center Institutional Review Board (IRB; IRB nos 161529 and 151721). NS, non-specific background.