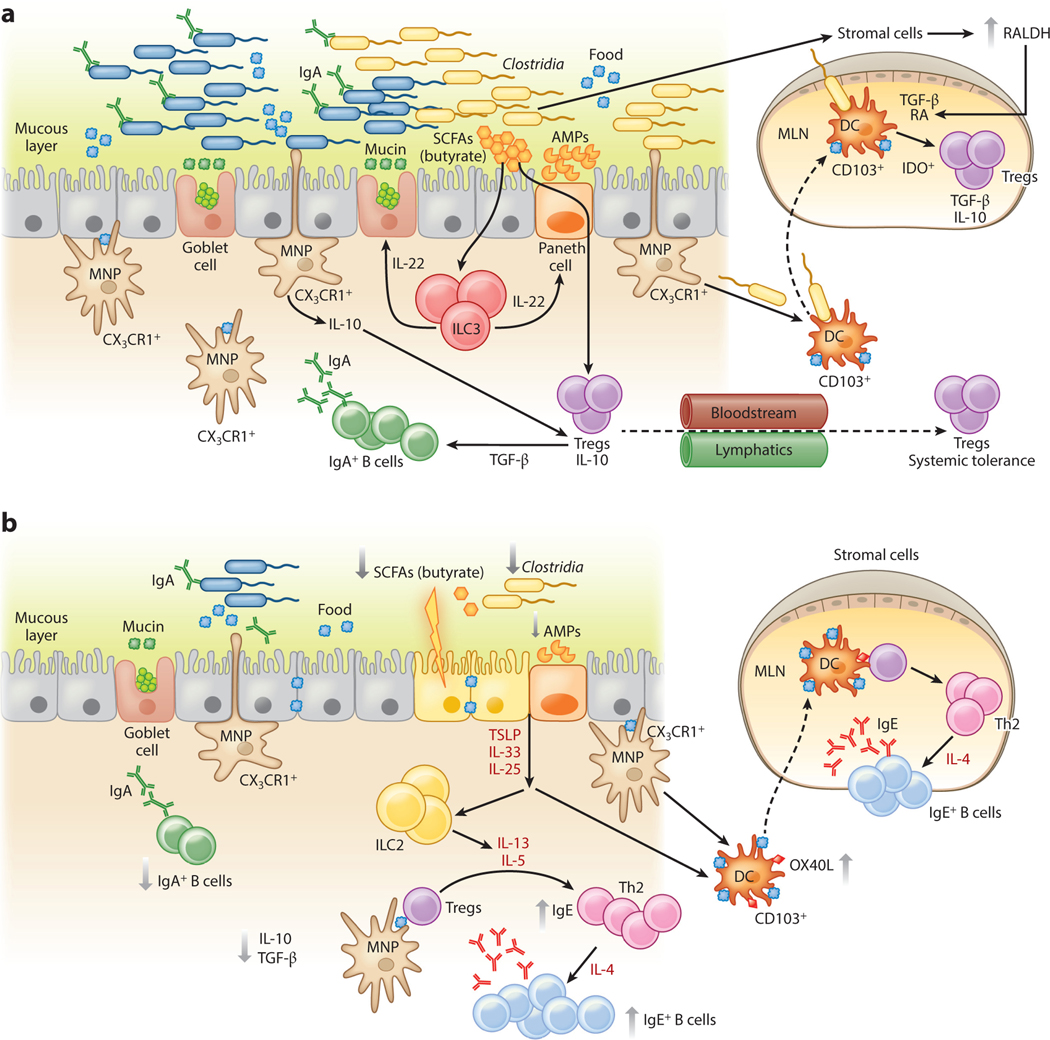
Figure 3.
An epithelial barrier in equilibrium with commensal bacteria is required for protection against allergic sensitization. (a) In healthy individuals, Clostridia (and possibly other allergy-protective commensal bacteria) maintain epithelial barrier integrity by stimulating ILC3s to produce IL-22. IL-22 promotes the production of mucus from goblet cells and antimicrobial peptides from Paneth cells and reduces the ability of food allergens to gain access to the systemic circulation. Clostridial metabolites, including SCFAs, directly induce Treg development. Treg-derived TGF-β favors local IgA antibody production, and circulating Tregs promote systemic tolerance. (b) Twenty-first-century lifestyle factors like antibiotics and high-fat, low-fiber diets promote microbial dysbiosis, predisposing to allergic sensitization. Depletion of Clostridia leads to loss of IL-22-dependent barrier functions and decreased concentrations of SCFAs, impairing barrier integrity and increasing allergen access. The stressed epithelium produces alarmins like TSLP, IL-33, and IL-25, which promote the generation of a Th2 immune response in part by stimulating ILC2s to produce Th2 cytokines. CD103+ DCs in a TSLP-rich microenvironment upregulate OX40L expression and traffic to MLNs or interact with lamina propria–resident T cells, where they stimulate the generation of antigen-specific Th2 cells producing IL-4 that triggers antibody class switching to IgE. Adapted from Reference 19 with permission from Cell Press. Abbreviations: AMP, antimicrobial peptide; DC, dendritic cell; ILC3, type 3 innate lymphoid cell; MLN, mesenteric lymph node; MNP, mononuclear phagocyte; RA, retinoic acid; SCFA, short-chain fatty acid; Treg, regulatory T cell.