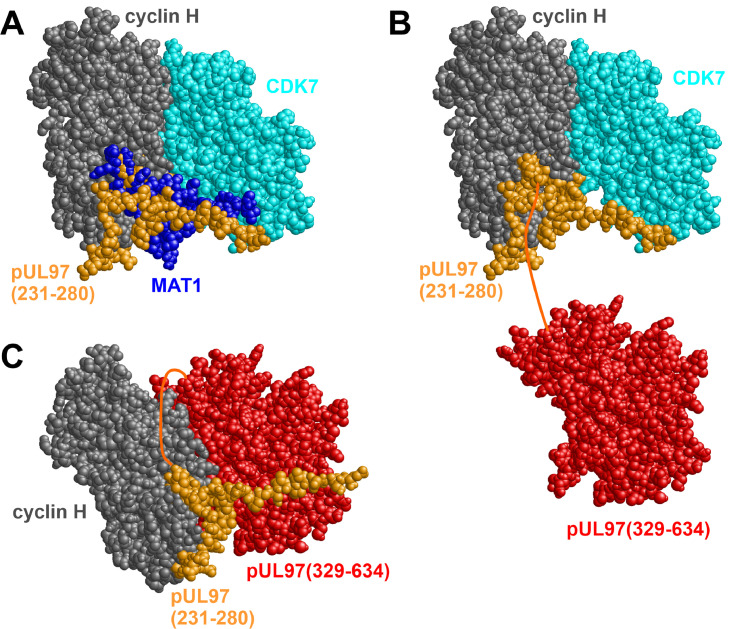
Fig. 1.
Structural model of the interaction between pUL97 and cyclin H. (A) Predicted binding site of pUL97(231-280) (orange) superimposed with the experimental cyclin H–CDK7–MAT1 complex structure (gray, cyan, and dark blue). This model suggests that pUL97(231-280) uses the same binding pocket as MAT1 for targeting the cyclin H–CDK7 complex. (B) Model of a ternary pUL97–cyclin H–CDK7 complex, in which pUL97 is attached to cyclin H exclusively through IF2 formed by the 231-280 sequence stretch. The pUL97 kinase domain (residues 329-634, marked in red) is connected to the complex by a nonstructured, flexible linker (residues 281-328, indicated as dark orange connecting line). (C) Model of a pUL97–cyclin H complex, in which pUL97 interacts with cyclin H both through IF2, pUL97(231-280) (orange), and the globular kinase domain IF1, pUL97(329-634) (red), thereby displacing CDK7.