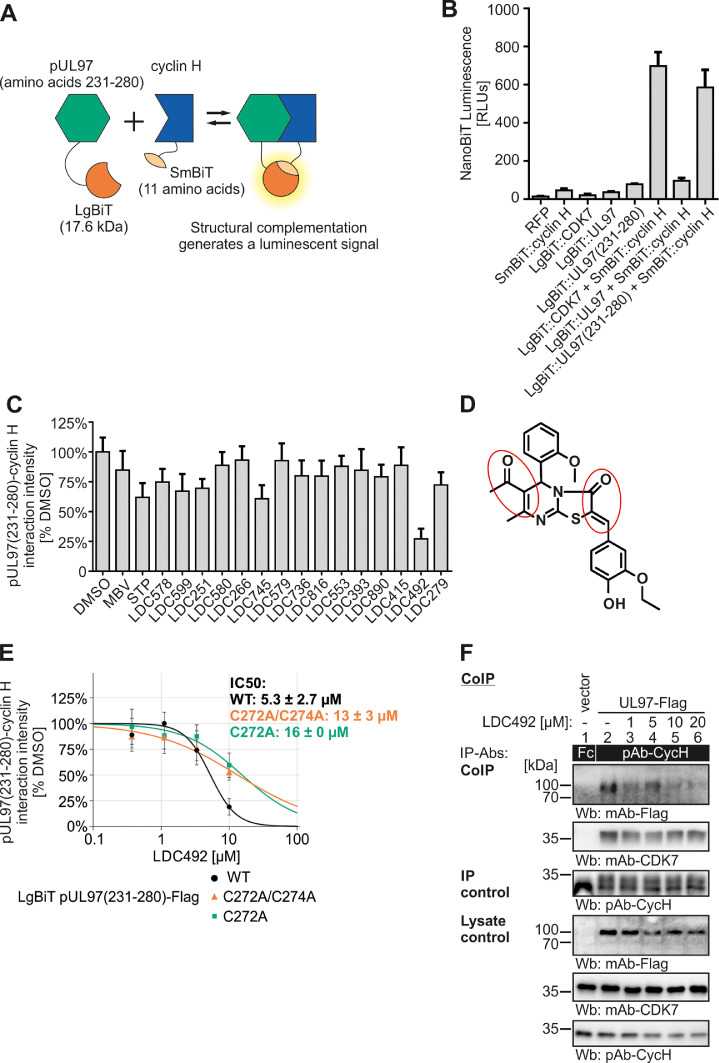
Fig. 10.
Measurements in the NanoBiT system demonstrate a warhead-mediated inhibition of pUL97(231-280)–cyclin H interaction. (A) Schematic depiction of the NanoBiT system. Proteins of interest are fused to LargeBiT (LgBiT) or SmallBiT (SmBiT) fragments of recombinant luciferase, to be coexpressed in transiently transfected cells. Interaction of proteins leads to the complementation of LgBiT and SmBiT, thereby generating a functional NanoLuc luciferase that converts a chemical substrate and thereby emits a chemoluminescence reporter signal. (B) NanoBiT test measurement of cells expressing red fluorescent protein (RFP), LgBiT::UL97, LgBiT::UL97(231-280), or LgBiT::CDK7 to evaluate the specificity of the system. Additionally, cells expressing LgBiT::CDK7, as well as the complete pUL97 (LgBiT::UL97) or solely the cyclin H interaction interface (LgBiT::UL97(231-280) were tested together with SmBiT::cyclin H to identify the most robust interaction pair. LgBiT::UL97(231-280)–SmBiT::cyclin H was used for further inhibitor analysis. (C) NanoBiT primary small-scale screening of potential inhibitors of the interaction LgBiT::pUL97(231-280) + SmBiT::Cyclin H. LgBiT:pUL97(231-280) and SmBiT::Cyclin H coexpressing cells were treated with a series of experimental compounds (LDC compounds, 10 µM), maribavir (MBV, 10 µM) or staurosporine (STP, 1 µM), the latter two as reference controls, before luciferase signal was measured. Signal intensities were normalized to the DMSO control. (D) The chemical structure of LDC492 is given and the α, β-unsaturated carbonyl groups are marked with red circles (E) 293T cells were transfected with LgBiT::UL97(231-280) WT, point mutants C272A/C274A or C274A, together with SmBiT::cyclin H, and treated with serial concentrations of LDC492 to determine IC50 values of pUL97(231-280)–cyclin H interaction (black curves). Replacement mutants C272A/C274A and C274A of LgBiT::pUL97(231-280) are depicted as orange or green curves, respectively. 293T cells were transiently transfected with plasmids encoding pUL97-Flag or empty vector control (F). Cells were lysed 2 d post-transfection and human cyclin H was immunoprecipitated; a rabbit Fc fragment served as a specificity control. A range of different concentrations of LDC492 or equal amounts of DMSO were added during cell lysis and CoIP. Subsequently, CoIP samples and lysate controls, taken before the addition of IP-Abs were then subjected to standard Wb analysis using the indicated antibodies.